Selective Targeting Agents for Mitochondria
a technology of selective targeting and mitochondria, applied in the field of mitochondrial selective targeting agents, can solve the problems of mitochondrial membrane “electron leakage”, apoptosis cell death, and the inability of the cell's natural antioxidants to compensate, so as to and prolong the survival of the patient.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0101]Materials. All chemicals were from Sigma-Aldrich (St Louis, Mo.) unless otherwise noted. Heparin, ketamine HCl and sodium pentobarbital were from Abbott Laboratories (North Chicago, Ill.). Dulbecco's modified Eagle medium (“DMEM”) was from BioWhittaker (Walkersville, Md.). Fetal bovine serum (FBS; <0.05 endotoxin units / ml) was from Hyclone (Logan, Utah). Pyrogen-free sterile normal saline solution was from Baxter (Deerfield, Ill.).
[0102]General. An moisture-sensitive reactions were performed using syringe-septum cap techniques under an N2 atmosphere and an glassware was dried in an oven at 150° C. for 2 h prior to use. Reactions carried out at −78° C. employed a CO2-acetone bath. Tetrahydrofuran (THF) was distilled over sodium / benzophenone ketyl; CH2Cl2, toluene and Et3N were distilled from CaH2. Me2Zn was purchased from Aldrich Company.
[0103]Reactions were monitored by thin layer chromatography (“TLC”) analysis (EM Science pre-coated silica gel 60 F254 plates, 250 μm layer th...
synthesis example i
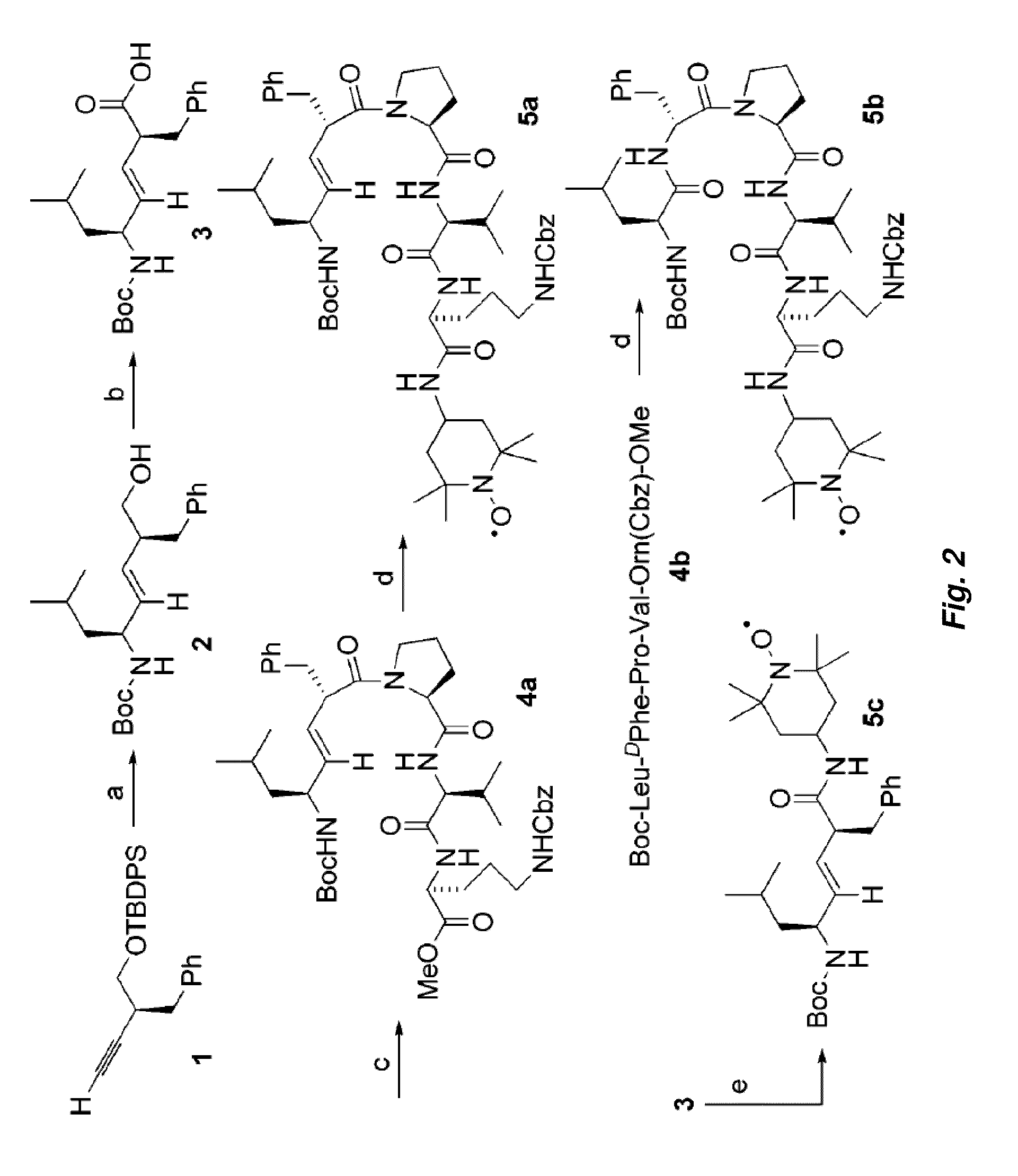
[0105]Prepared as a colorless oil (FIG. 2, compound 1) according to the literature procedure, see Edmonds, M. K. et al. Design and Synthesis of a Conformationally Restricted Trans Peptide Isostere Based on the Bioactive Conformations of Saquinavir and Nelfinavir J. ORG. CHEM. 66:3747 (2001); see also Wipf, P. et al., Convergent Approach to (E)-Alkene and Cyclopropane Peptide Isosteres ORG. LETT. 7:103 (2005); see also Xiao, J. et al., Electrostatic versus Steric Effects in Peptidomimicry: Synthesis and Secondary Structure Analysis of Gramicidin S Analogues with (E)-Alkene Peptide Isosteres J. AM. CHEM. SOC. 127:5742 (2005).
[0106]A solution of 2.20 g (5.52 mmol) of compound 1 (FIG. 2) in 20.0 mL of dry CH2Cl2 was treated at room temperature with 1.85 g (7.17 mmol) of Cp2ZrHCl. The reaction mixture was stirred at room temperature for 5 min, CH2Cl2 was removed in vacuo and 20.0 mL of toluene was added. The resulting yellow solution was cooled to −78° C. and treated over a period of 30 ...
example i
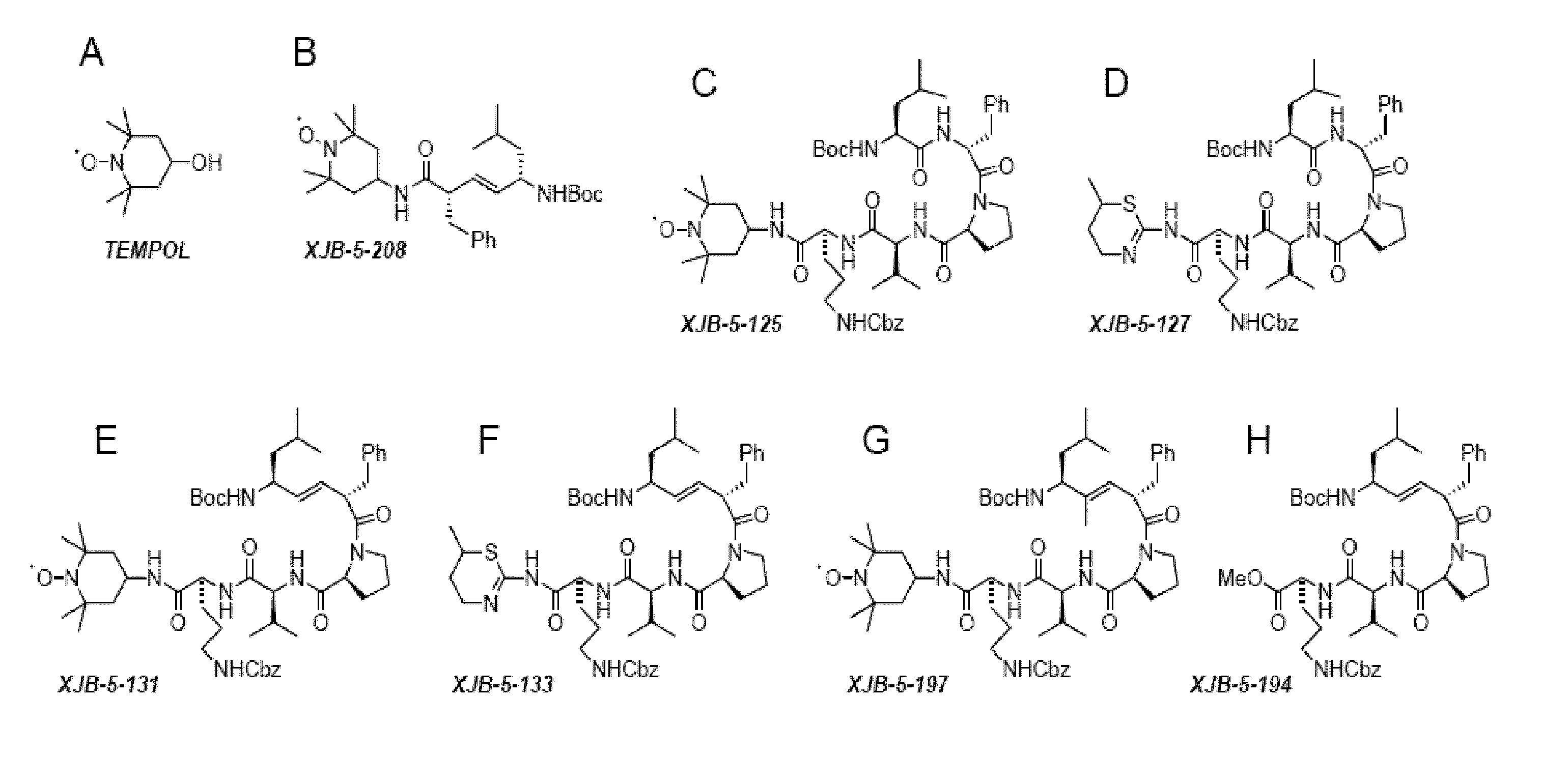
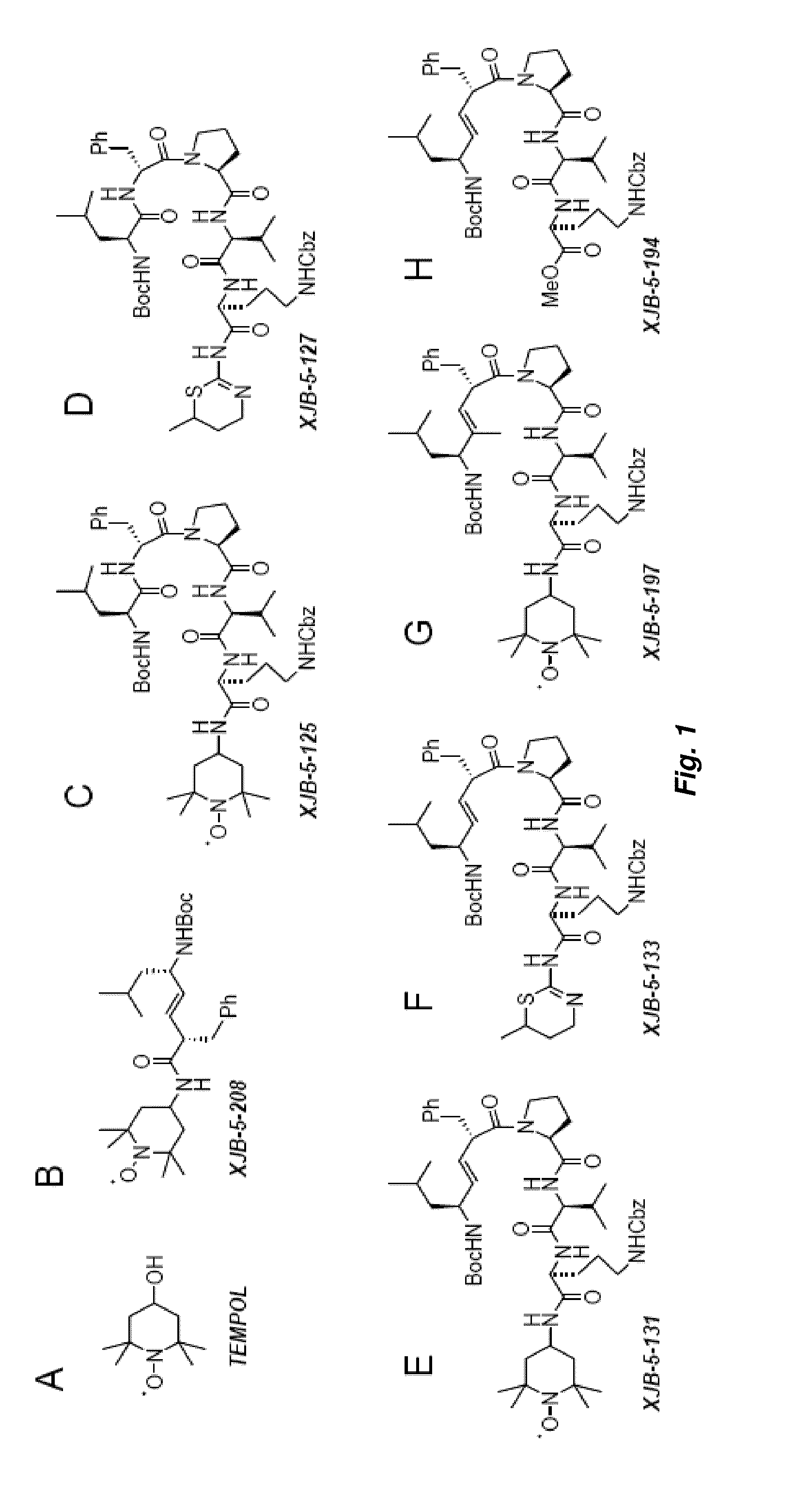
[0143]Selective delivery of TEMPO to mitochondria could lead to therapeutically beneficial reduction of ROS; therefore, investigation of the use of conjugates of 4-amino-TEMPO (“4-AT”) was explored. In order to selective target the mitochondria, a targeting sequence using the membrane active antibiotic Gramicidin S (“GS”) as well as corresponding alkene isosteres, shown in FIGS. 1 and 2. Accordingly, using the Gramicidin S peptidyl fragments and alkene isosteres as “anchors,” the TEMPO “payload” could be guided into the mitochondria.
[0144]The Leu-DPhe-Pro-Val-Orn fragment of hemigramicidin was used as a targeting sequence. Alkene isosteres such as (E)-alkene isosteres of Gramicidin S (i.e., hemigramicidin) were used as part of the targeting sequence. See FIG. 3 for the synthetic pathway for (E)-alkene isosteres and compound 3 for the corresponding chemical structure. The (E)-alkene as depicted in compound 2 of FIG. 2 was then oxidized in a multi-step process to yield the compound as...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com