Diagnostics and treatments of periodontal disease
a multi-meric cell and disease technology, applied in the field of diagnosis and treatment of periodontal disease, can solve the problems of limited application to date, major public health problems, and cell-associated trypsin-like proteolytic activities of i>p. gingivalis /i> have not been characterised to date,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
(1) Preparation of Antigen
A. Anion Exchange and Affinity Chromatography
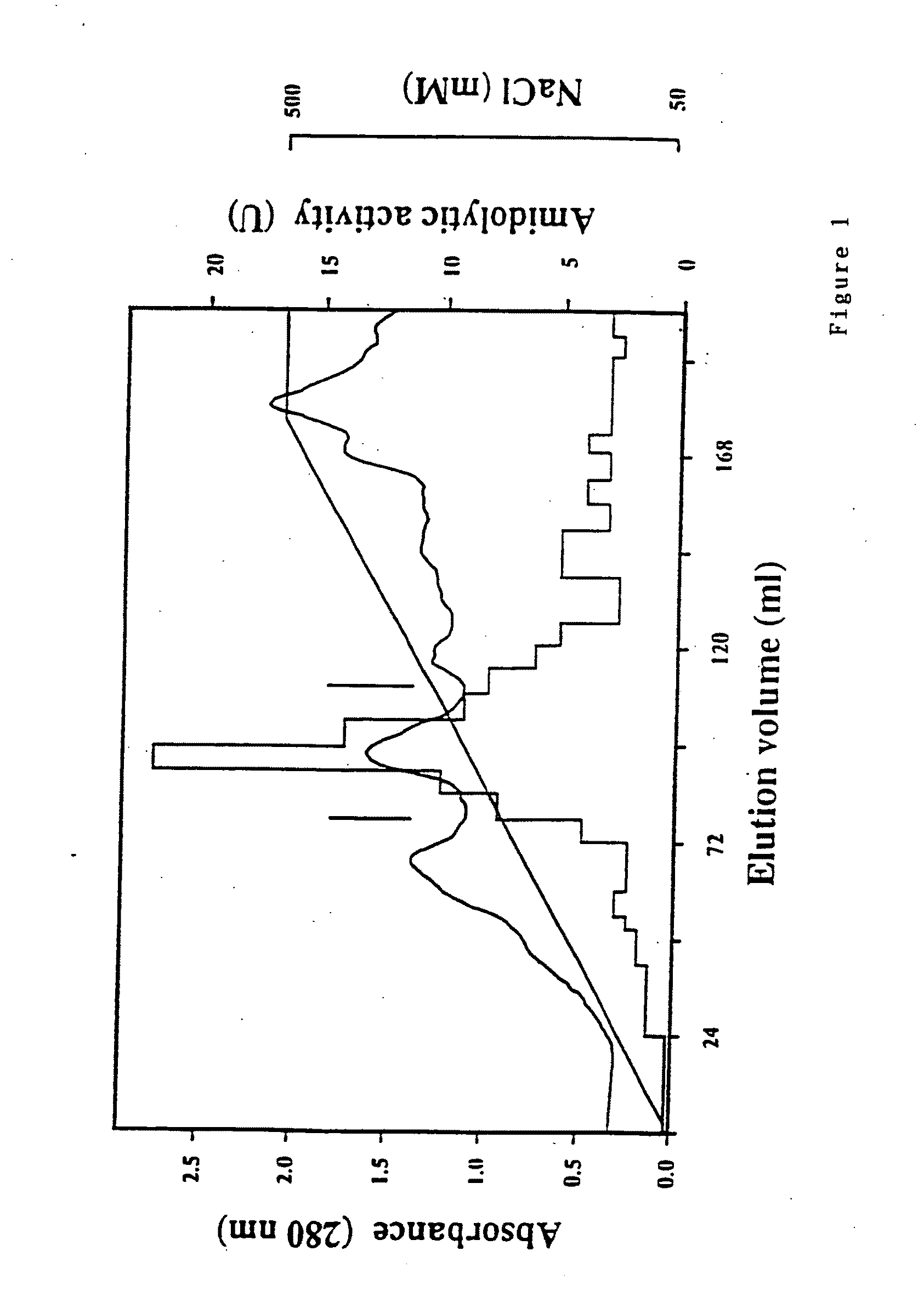
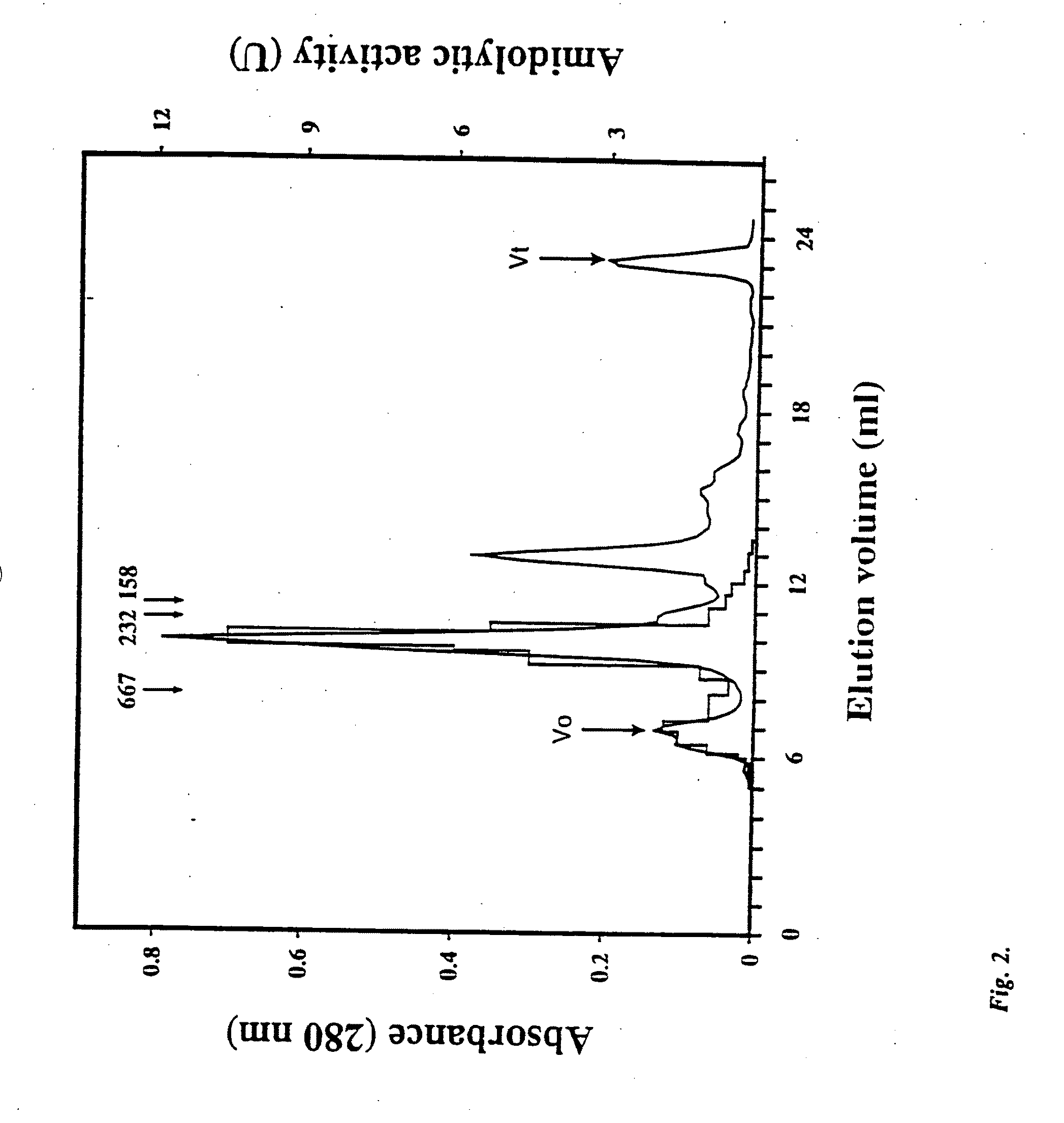
[0076]P. gingivalis W50 was grown anaerobically at 37° C. on lysed horse blood agar and in modified BM media containing 1 μg / ml hemin. Bacteria were maintained on lysed horse blood plates by routine passage (P. gingivalis was grown to late logarithmic phase and the cells harvested by centrifugation (5,000×g, 20 min, 4° C.) and then resuspended in 160 ml TC buffer (20 mM Tris-HCl pH 7.4 and 5 mM CaCl2) containing 50 mM NaCl and subjected to mild sonication using a Branson Sonifier 250 with an output control of 3 and a 50% duty cycle for 15 min at 4° C. The sonicate was centrifuged (100,000×g, 30 min, 4° C.) and the supernatant filtered (0.22 μm) prior to anion-exchange FPLC. The sonicate was applied to an anion-exchange column (Hiload XK 16 / 10 Q Sepharose, Pharmacia-LKB) cooled to 4° C., in multiple injections using a 50 ml superloop (Pharmacia-LKB). The sample was eluted using a linear gradient from 0-100% buffer...
example 2
[0092]Methods and compounds for vaccine formulations related to PrtR-PrtK.
[0093]This embodiment of the present invention is to provide PrtR-PrtK protein to be used in as an immunogen in a prophylactic and / or therapeutic vaccine for active immunization to protect against or treat infections caused by P. gingivalis. For vaccine purposes, an antigen of P. gingivalis comprising a bacterial protein should be immunogenic, and induce functional antibodies directed to one or more surface-exposed epitopes on intact bacteria, wherein the epitope(s) are conserved amongst strains of P. gingivalis.
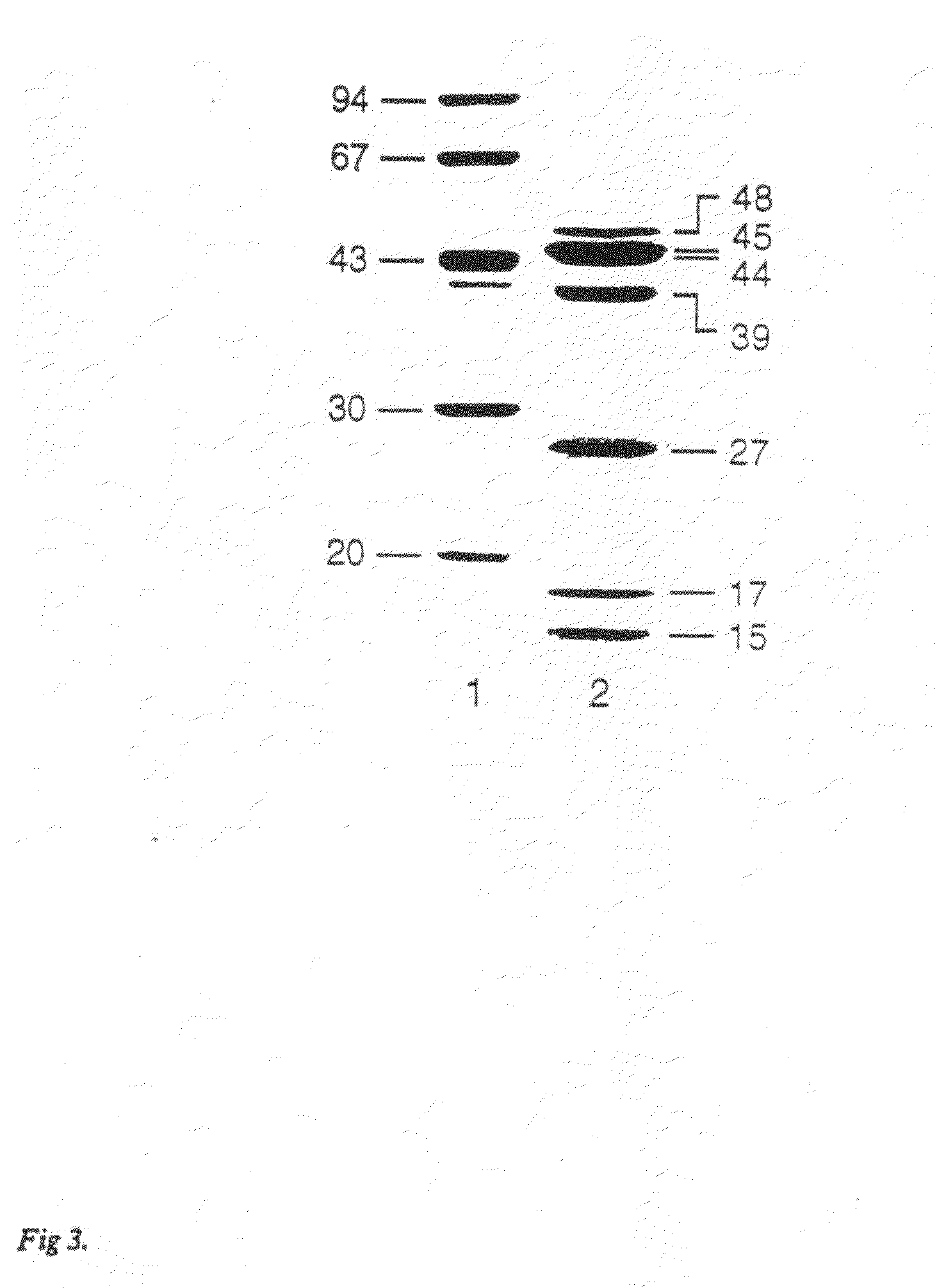
[0094]In one illustration of the PrtR-PrtK protein having the properties desirable of a vaccine antigen, the protein was purified from P. gingivalis using the method described herein in Example 1. Mice were immunized with the purified inactivated PrtR-PrtK protein (25 μg) with adjuvant (20 ug of QS21) two times at four week intervals. The purified PrtR-PrtK was inactivated by air oxidation. Blood from...
example 3
[0099]Protective Efficacy of Immunisation with the PrtR-PrtK Complex in an Animal Model.
[0100]Various preparations of purified P. gingivalis proteins were tested in the mouse abscess model. This model is loosely based on the methods described by Kesavalu et al (1992) [Infect Immun 60:1455-1464]. A typical experiment is outlined below. Briefly BALB / c mice were obtained from ARC (Perth, Australia) and were immunised subcutaneously in the scruff of the neck with the preparations and doses according to Table 5 before challenge with live P. gingivalis strain W50, which was given at 10 weeks of age. Mice were given 2 doses of vaccine at 4 and 1 weeks before challenge. Formalin killed P. gingivalis W50 cells were prepared by incubating an aliquot of cells in 0.5% (vol / vol) of buffered formal saline overnight at 4° C. The chloroform extract of P. gingivalis was prepared as detailed in Example 2. Purification of PrtR-PrtK complex was performed as detailed in Example 1. The PrtR-PrtK domains ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| flow rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com