New drug for inhibiting aggregation of proteins involved in diseases linked to protein aggregation and/or neurodegenerative diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of a Novel Class of Compounds for Inhibiting Protein Aggregation
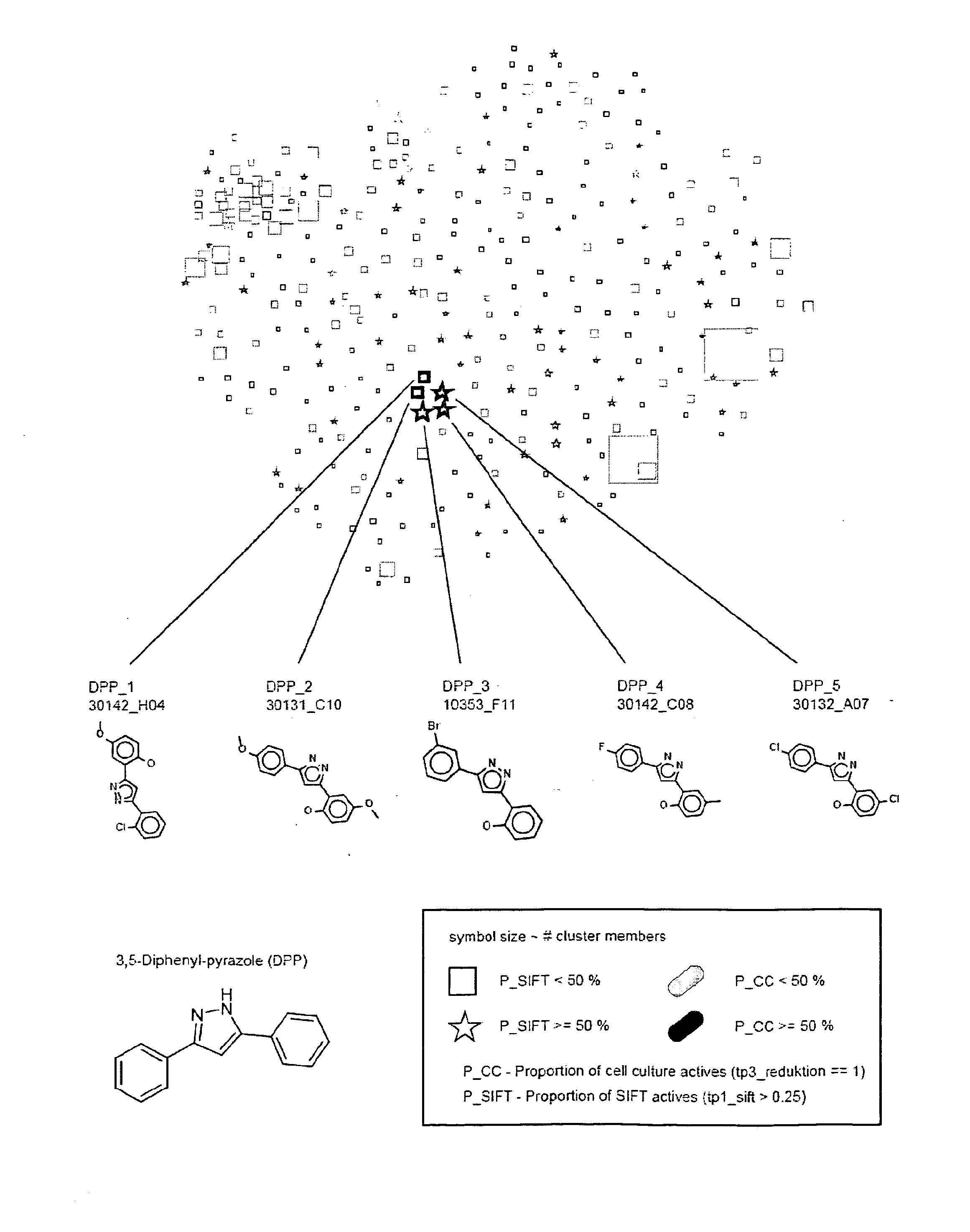
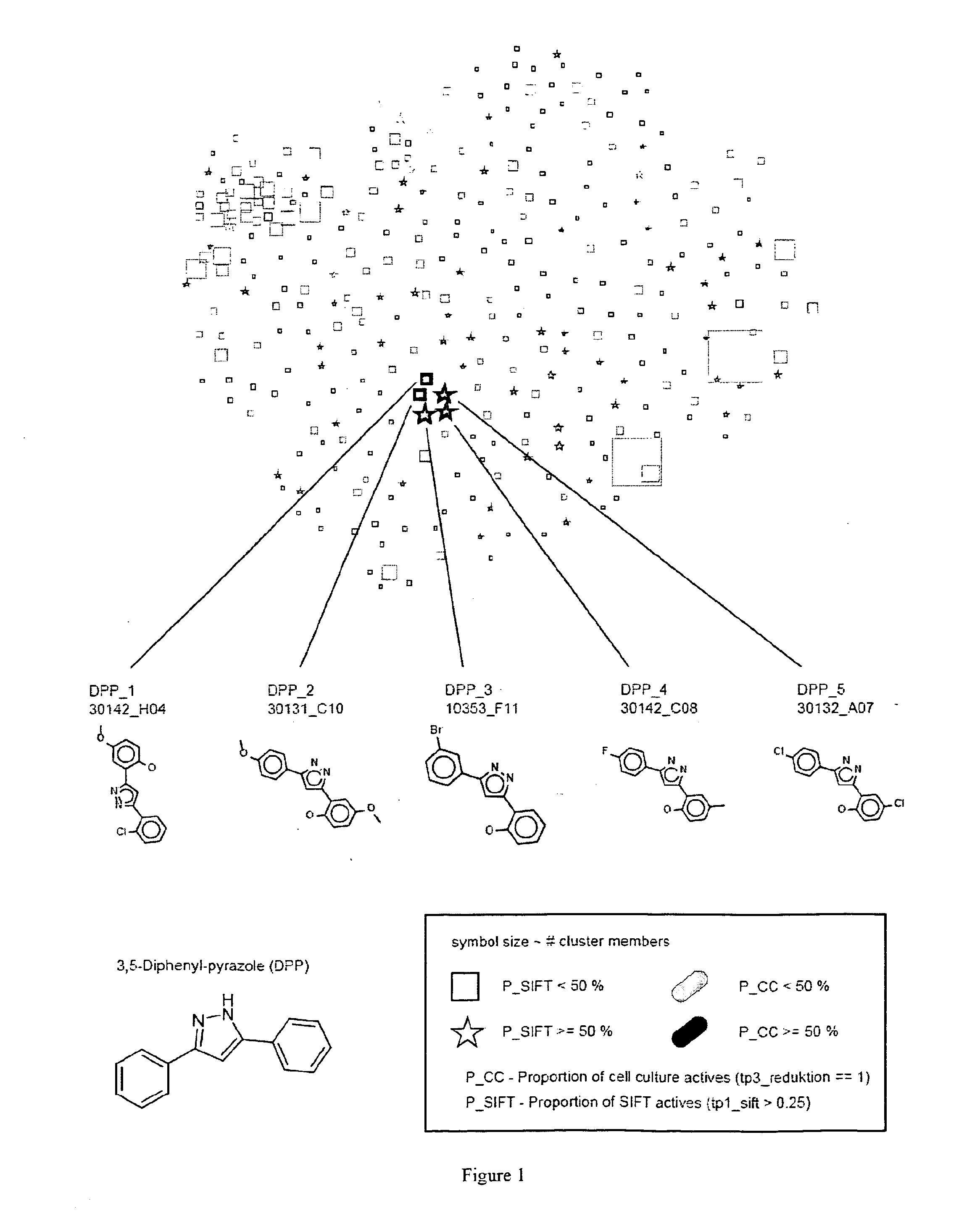
[0196]Two subsets of the commercial compound library DIVERSet (ChemBridge Corp., San Diego, Calif., USA), each containing 10.000 compounds, and called DIVERSet 1 and 2 by us, have been screened for inhibitors of prion propagation using the 2D-SIFT anti-prion assay (Bertsch et al. 2005) and a cell culture model of prion disease. In both assays primary hits were obtained by testing compounds at a single concentration and subsequently verified in dilution series. Additionally, cell culture hits were tested using another cell line.
2DSIFT Screening
[0197]To test the inhibitory effect of drugs on the association between PrPC and PrPSc in a high-throughput and high-content screening assay, we applied the “Scanning for Intensely Fluorescent Targets” (SIFT-) technique, which utilises an inverted dual color confocal microscope setup with single photon detectors for two colors of fluorescent light. Samples are prepar...
example 2
Synthesis of New Drugs for Inhibiting Aggregation Under Medicinal-Chemical Aspects
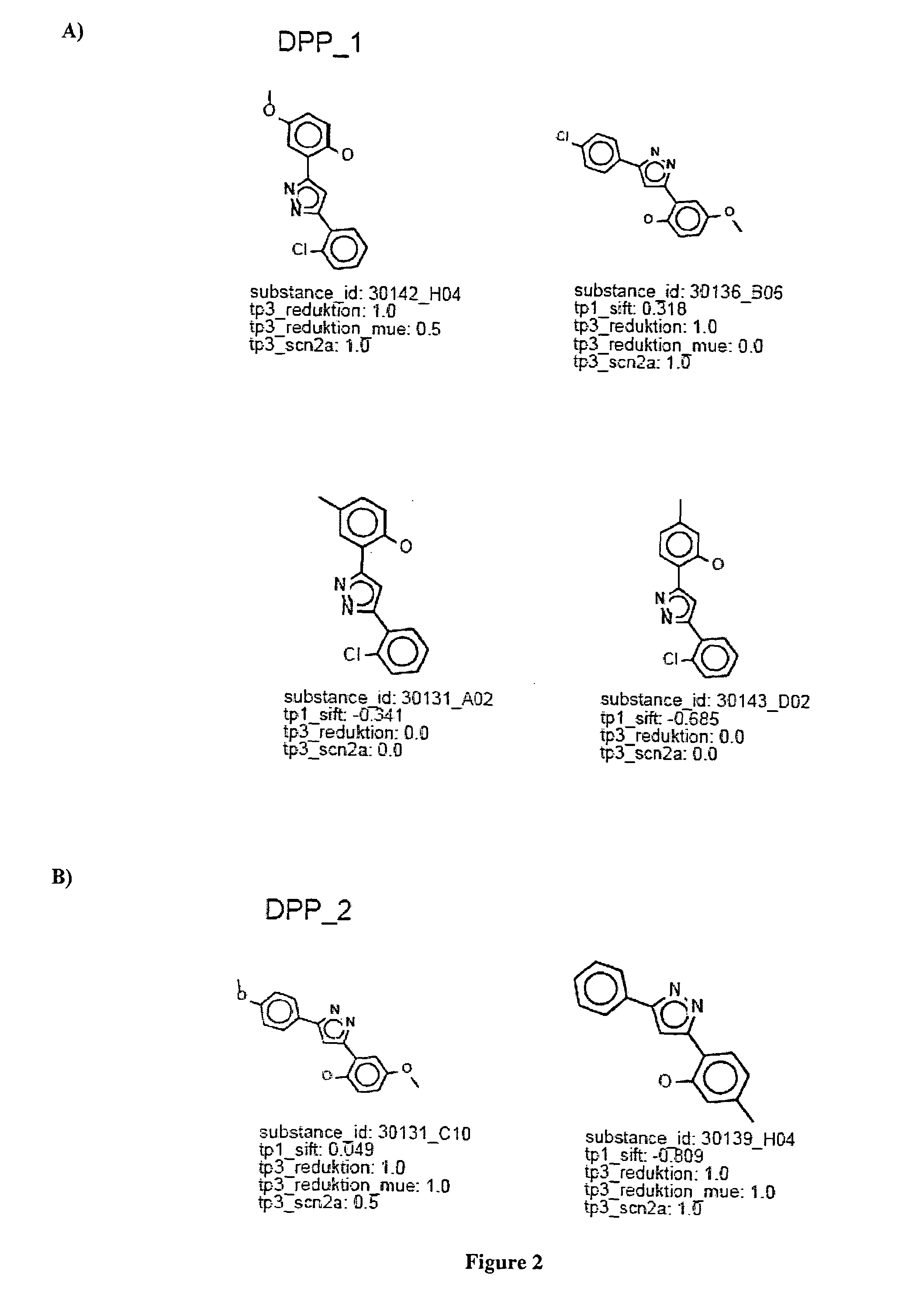
[0206]Based on the discovery of the new lead structure as described above, a number of further substances were synthesised by selective substitution of different substituents, as outlined below.
(E)-1-(3,4-Dimethoxyphenyl)-3-(3-fluorophenyl)-2-propene-1-one (1) [Nam et al., 2004]
[0207]A solution of 3,4-dimethoxyacetophenone (1.8 g, 10 mmol), 3-fluorobenzadehyde (1.24 g, 10 mmol), NaOH (50 mg, 1.25 mmol) and Ba(OH)2.8H2O (100 mg, 0.32 mmol) in methanol (10 ml) was stirred at room temperature for 24 h. The reaction was cooled to +4° C., resulting precipitate was collected by filtration, recrystallized from methanol and dried to provide 1 (1.65 g, 58%) as a yellow powder.
2,3-Dibromo-1-(3,4-dimethoxyphenyl)-3-(3-fluorophenyl)-propan-1-one (2) [Harris et al., 1977]
[0208]To a solution of 1 (715 mg, 2.5 mmol) in chloroform (11 ml) was added dropwise a solution of bromine (400 mg, 2.5 mmol) in chloroform (4 ml)...
example 3
Material and Methods Used
Compound Libraries
[0246]The libraries screened contain 10.000 compounds each and are called DIVERSet1 and DIVERSet2 by us, because they cover only a part of the larger DIVERSet library (ChemBridge Corp., San Diego, Calif.). DIVERSet is a collection of rationally selected, diverse, drug-like small molecules. The compounds were supplied in dimethyl sulfoxide (DMSO) solution and on 96-well microtiter plates. A database containing molecular structures and some physico-chemical data for each of the compounds is available at www.chembridge.com.
Production of Recombinant Mouse PrP 23-231
[0247]Recombinant PrP 23-231 was produced and purified essentially as described by Liemann et al. (1998), except that for bacterial expression BL21DE3 RIL E. coli cells (Novagen) were transformed with plasmid pET17b-MmPrP23-231WT31 for mouse PrP23-231. Also, the bacteria were grown to an optical density of 0.5 before protein production was induced by addition of 1 mM IPTG and cells h...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric charge | aaaaa | aaaaa |
| Capacitance | aaaaa | aaaaa |
| Angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com