Polylactide resin and preparation method thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
[0091]0.2 g (0.49 mmol) of Sn(Oct)2 (Aldrich Co.) and 0.36 g (1.0 mmol) of the compound of Chemical Formula 4 (TCI Inc.) were put into a 100 mL flask, 30 mL of toluene was added thereto, and the mixture was stirred at 100° C. for 1 hour. Then, after the solvent was removed under vacuum, the resulting product was washed with a heptane solvent and dried to give 0.36 g of organometallic complex A.
synthesis example 2
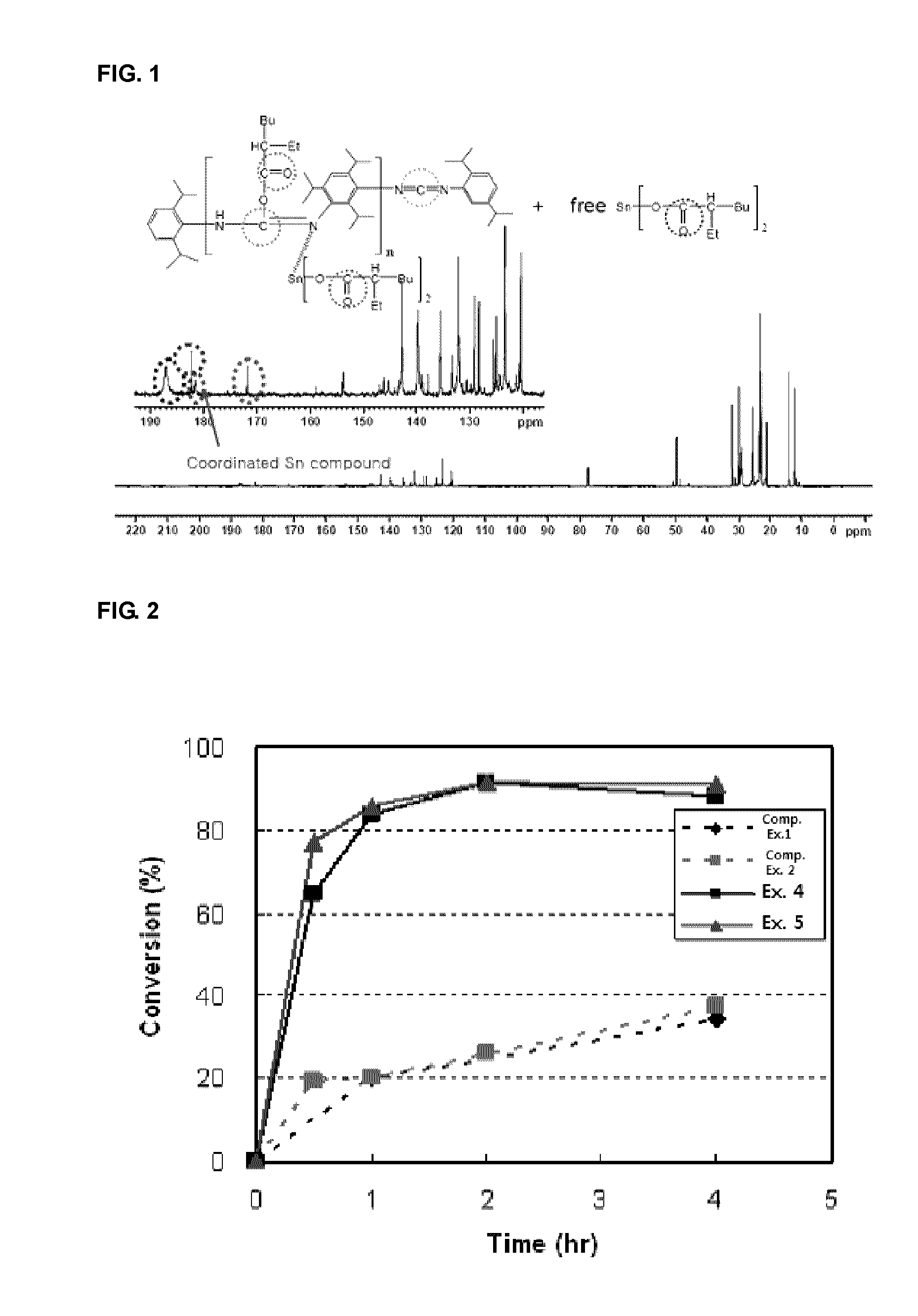
[0092]0.2 g (0.49 mmol) of Sn(Oct)2 (Aldrich Co.) and 0.36 g of the compound of Chemical Formula 5 (Rhein Chemie Inc.) were put into a 100 mL flask, and in the same manner as in Synthesis Example 1, 0.4 g of organometallic complex B was obtained.
[0093]FIG. 1 is 13C NMR spectrum of organometallic complex B. Referring to FIG. 1, in the reaction of the Sn(Oct)2 catalyst and the compound of Chemical Formula 5, three peaks for a carbonyl group are shown at δ 188, 183, and 182 ppm, respectively. The peak at δ 183 ppm, which is very sharp, can be assigned to the one corresponding to the Oct-H acid compound coupled with the compound of Chemical Formula 5. The broad peak at δ 188 ppm corresponds to the one for free Sn(Oct)2 and the broad peak at δ 182 ppm can be assigned to the one corresponding to the organometallic complex coordinated by the compound of Chemical Formula 5.
synthesis example 3
[0094]0.2 g (0.49 mmol) of Sn(Oct)2 (Aldrich Co.) and 0.12 g (1.0 mmol) of the compound of Chemical Formula 6 (TCI Inc.) were put into a 100 mL flask, 30 mL of toluene was added thereto, and the mixture was stirred at 100° C. for 1 hour. Then, after the solvent was removed under vacuum, the resulting product was washed with a heptane solvent and dried to give 2.5 g of organometallic complex C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com