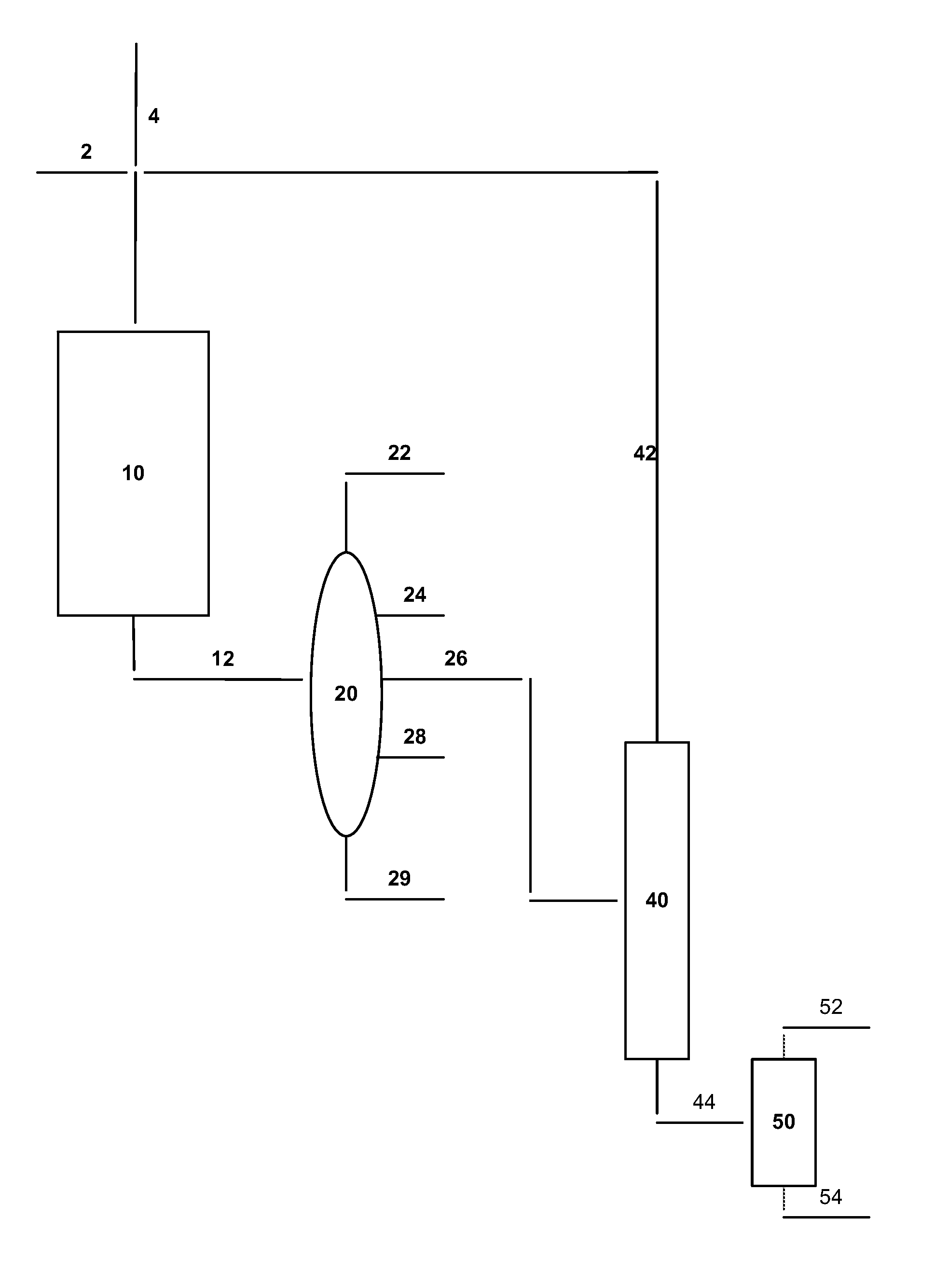
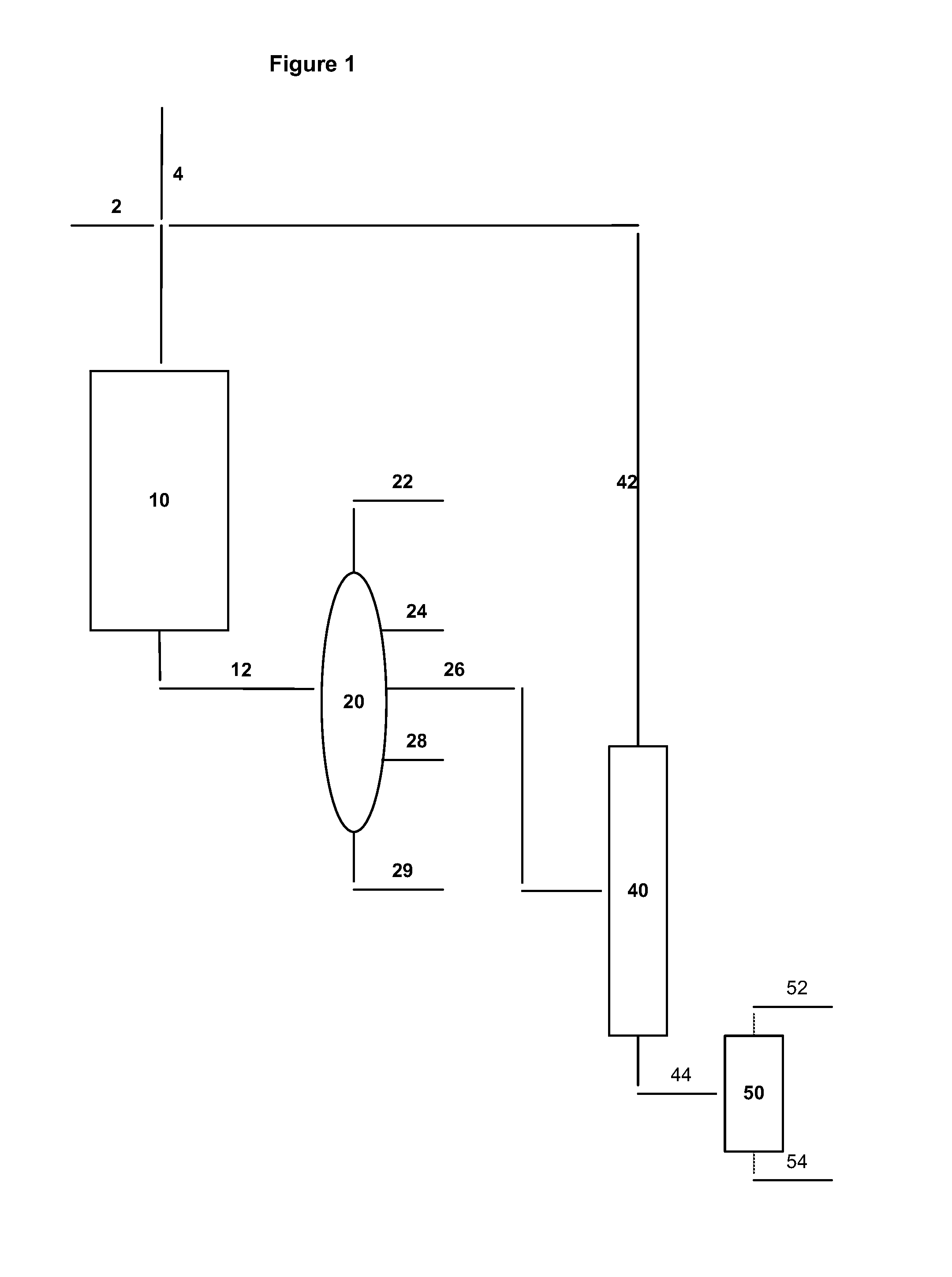
Process for the production of para-xylene
a production process and para-xylene technology, applied in the field of para-xylene production, can solve the problems of difficult separation of csub>8 /sub>aromatics, general undesirabledealkylation and hydrocracking during catalytic reforming,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0077]A naphtha feedstock comprising greater than 10% C8 paraffinic hydrocarbons, with an ASTM D-2887 simulated distillation shown in Table 1, was used as feed for the process of the invention and the following comparative examples (IBP=initial boiling point, EP=end boiling point). The feedstock composition was characterized by API, RON, and GC analysis with results given in Table 2, where B stands for benzene, T for toluene, X for all three xylene isomers and EB for ethylbenzene while PX / MX stands for the yield ratio of para-xylene to meta-xylene.
TABLE 1ASTM D-2887 simulated distillation of the feedVol. %Temperature, ° F.IBP771016830218502467027390302EP331
TABLE 2Other properties of the feedAPI57.7RON65.9C5+, wt. %99.9Benzene, wt. %0.5Toluene, wt. %1.7Ethylbenzene, wt. %1.7m-Xylene, wt. %1.1p-Xylene, wt. %0.5o-Xylene, wt. %1.1Total BTX + EB, wt. %4.9PX / MX0.46Total C8, wt. %25.4C8 paraffins, wt. %13.7C8 naphthenes, wt. %7.3
example 2
Comparative
[0078]The naphtha feed described in Example 1 was contacted in a fixed-bed reactor containing a commercial amorphous reforming catalyst comprising platinum with a rhenium promoter on an alumina support. The reaction conditions included the temperatures of 885, 895, 905 and 915° F., a pressure of 350 psig, a liquid hourly space velocity (LHSV) of 1.5 hr−1 and a molar ratio of hydrogen to hydrocarbon of 5:1.
[0079]The yield of C5+ liquid, its RON and other properties as well as the hydrogen production obtained under the aforementioned conditions are listed in Table 3, where HC stands for hydrocarbons and H2 / HC for the molar ratio of hydrogen to hydrocarbon at the reactor inlet. A PX / MX ratio of about 0.41 was obtained for all the products at these four temperatures.
TABLE 3Properties of reforming products obtained from acommercial reforming catalyst comprising platinumwith a rhenium promoter on an alumina support.Pressure, psig350LHSV, hr−11.5H2 / HC5:1Temperature, ° F.88589590...
example 3
Invention
[0080]The naphtha feed described in Example 1 was contacted in a fixed-bed reactor containing a ZSM-5 zeolite based catalyst composited with 30 wt. % alumina binder material. The ZSM-5 had a SiO2 / Al2O3 molar ratio of about 500 and was ion exchanged to the ammonium form before incorporating in a 70 wt. % zeolite on 30 wt. % alumina extrudate. The extrudate was impregnated with 0.8 wt. % Pt, 0.38 wt. % Re, 0.35 wt. % Na and 0.3 wt. % Mg by an incipient wetness procedure to make the final catalyst. The reaction conditions included the temperatures of 865, 875, 885, 895, 905 and 915° F., a pressure of 80 psig, a liquid hourly space velocity (LHSV) of 1.0 hr−1 and a molar ratio of hydrogen to hydrocarbon of 2:1.
[0081]The yield of C5+ liquid, its RON and other properties as well as the hydrogen production obtained under the aforementioned conditions are listed in Table 4. The PX / MX ratio of the products produced over the ZSM-5 zeolite based catalyst in this example ranged from 1....
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallite size | aaaaa | aaaaa |
| molar ratio | aaaaa | aaaaa |
| weight ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com