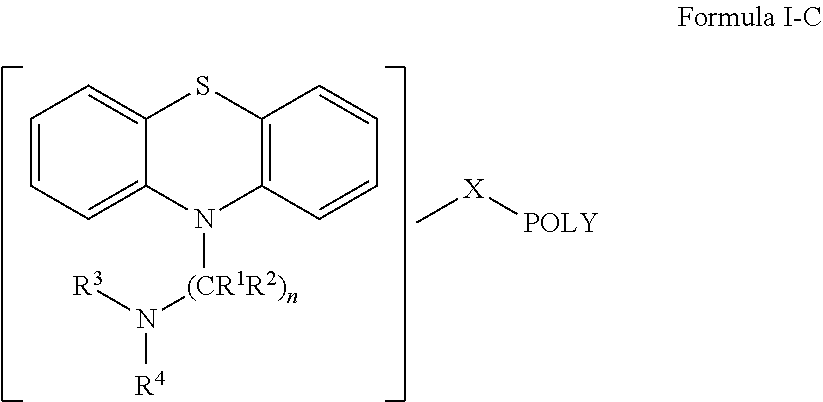
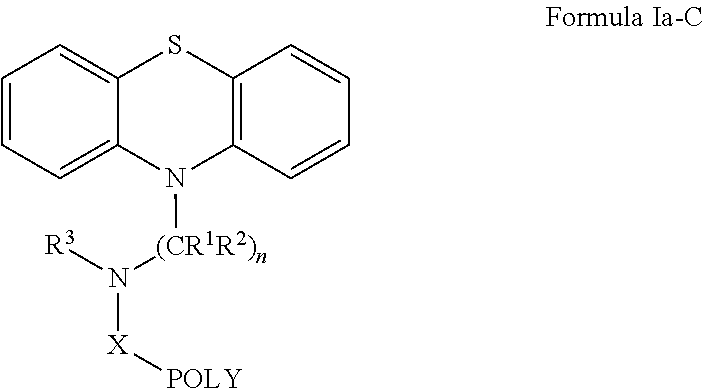
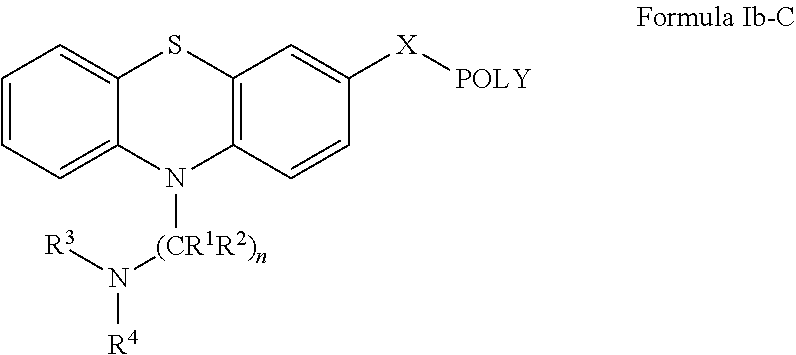
Oligomer-Phenothiazine Conjugates
a technology of phenothiazine and conjugates, which is applied in the field of chemically modified phenothiazine, can solve the problems of large unmet need for developing novel phenothiazine compounds, and the incidence of venous thrombosis at the injection si
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Small PEG Promethazine Conjugates
[0142]Scheme 1: Synthesis of mPEGn-N-Promethazine conjugates.
[0143]Promethazine, 1-chloroethyl chloroformate, dichloroethane (DCE), phosphorous oxychloride (POCl3), sodium borohydride (NaBH4), sodium hydride (NaH), and N,N-dimethylformamide (DMF) were from Sigma-Aldrich (St Louis, Mo.). mPEGn-OMs and mPEGn-Br were from Sai Chemicals (India). Sodium carbonate (Na2CO3), sodium bicarbonate (NaHCO3), sodium sulfate (Na2SO4), potassium carbonate (K2CO3), sodium chloride (NaCl), and sodium hydroxide (NaOH) were from EM Science (Gibbstown, N.J.). DCM was distilled from CaH2.
[0144]Desalting of Promethazine-HCl: The Sigma-Aldrich promethazine.HCl (10 g, 31.2 mmol) was dissolved in H2O (250 mL) in a 500-mL flask. Na2CO3 (8.2 g, 78 mmol) was added in one portion. The salt was first dissolved in water and then a thick oil-like product appeared sticking to the stirring bar. DCM (50 mL) was added to dissolve the product and the solution became clear a...
example 2
[0166]Binding to Histamine Receptors
[0167]The receptor binding affinity of promethazine (parent) and N- and O-PEG derivatives are evaluated using radioligand binding assays in membranes prepared from CHO cells that express the recombinant human H1, H2, H3 or H4 histamine receptors. Competition binding experiments are conducted by incubating membranes with a fixed concentration of radioligand in the presence of variable concentrations of test compounds. The radioligands used are specific for each receptor subtype and the assay conditions are described in Table 2. Following incubations, the membranes are washed, and the bound radioactivity is measured. Non-specific binding is measured in the presence of excess cold ligand and subtraction of this value from the total binding yields the specific binding at each test compound concentration. IC50 values are obtained from non-linear regression analysis of dose-response curves and are calculated only for those compo...
example 3
Scale up of mPEG4-N-promethazine
[0173]Materials: Promethazine hydrochloride, 1-chloroethyl chloroformate were purchased from Sigma-Aldrich (St Louis, Mo.). mPEG4-Br was received from India Sai CRO. Sodium bicarbonate (NaHCO3), sodium sulfate (Na2SO4), sodium chloride (NaCl), Potassium carbonate (K2CO3), Sodium hydroxide (NaOH), and hydrochloride acid (HCl) were purchased from EM Science (Gibbstown, N.J.). Toluene, dichloroethylene (DCE), dichloromethane (DCM), and other organic solvents were used as they purchased.
Desalting Promethazine
[0174]The reaction was carried out in NaHCO3 / DCM. The product was solidified in high vacuo drying.
Demethylation and Product Precipitation:
[0175]The desalted promethazine (13.16 g, 46.3 mmol) was added to a 1000-mL flask. Toluene (110 mL) and DCE (100 mL) were added and azeotropically distilled while under 45° C. then followed with distillation under high vacuum for an additional 15 min. DCE (100 mL) was added and after a homogenous solution was obtain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com