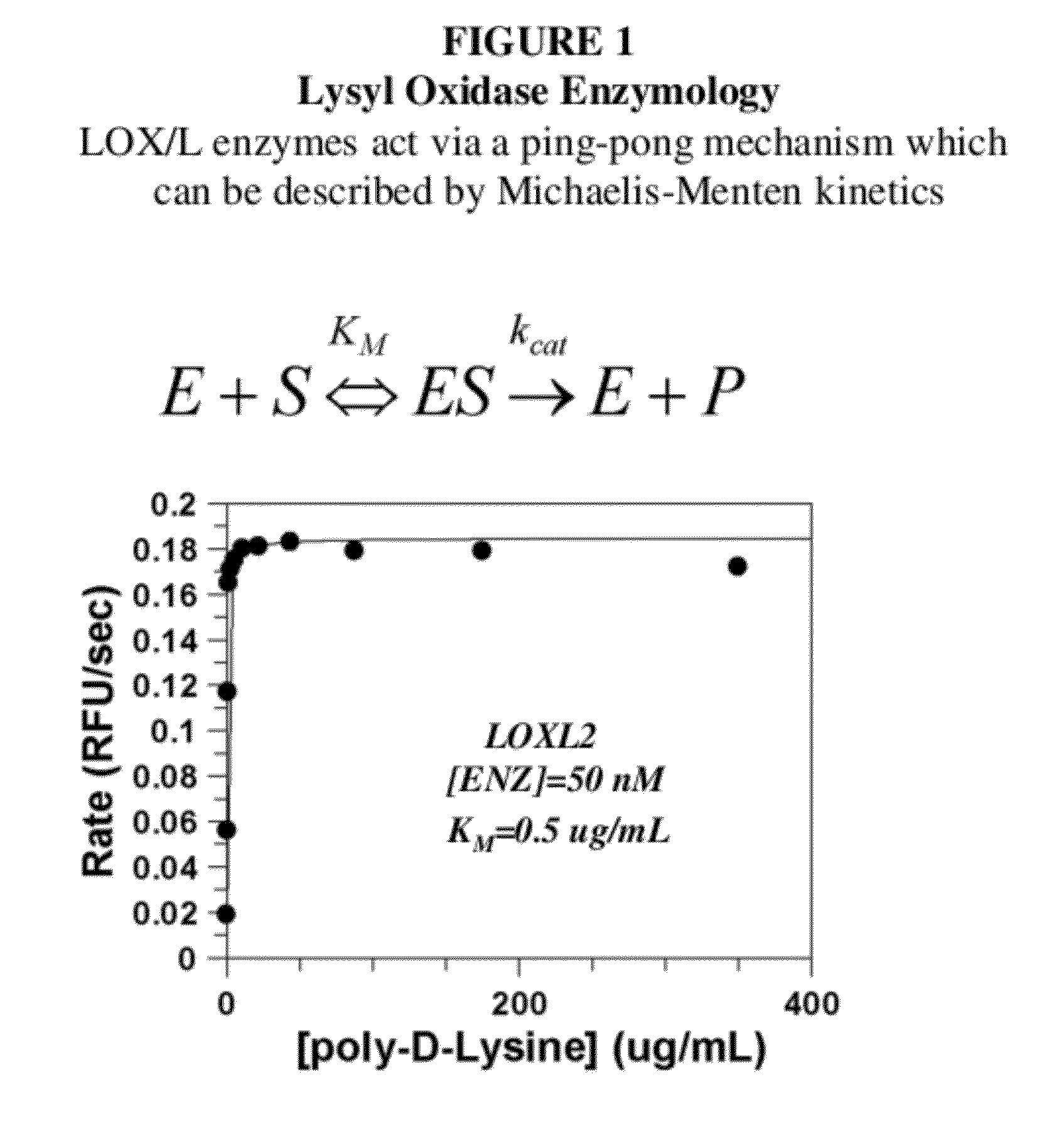
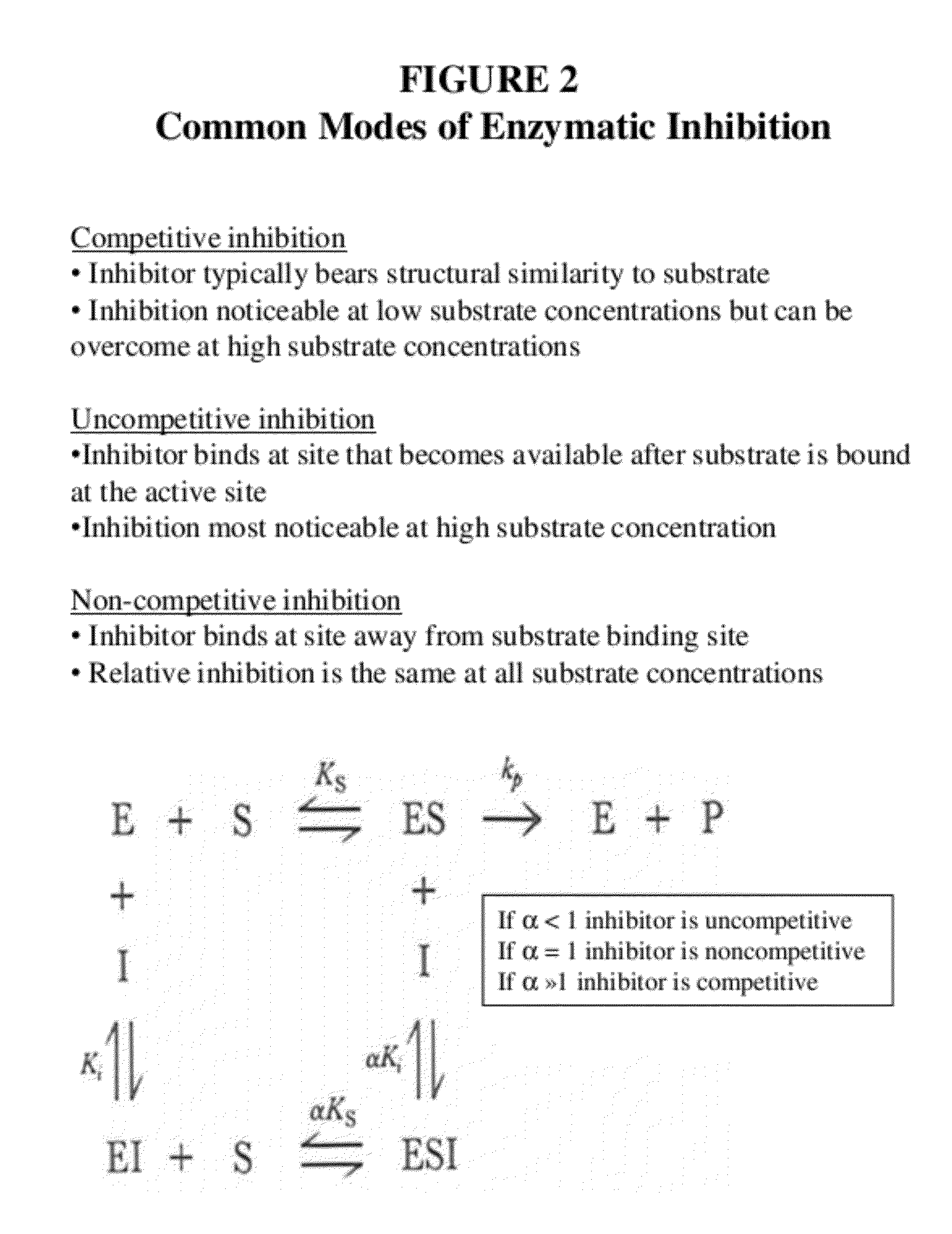
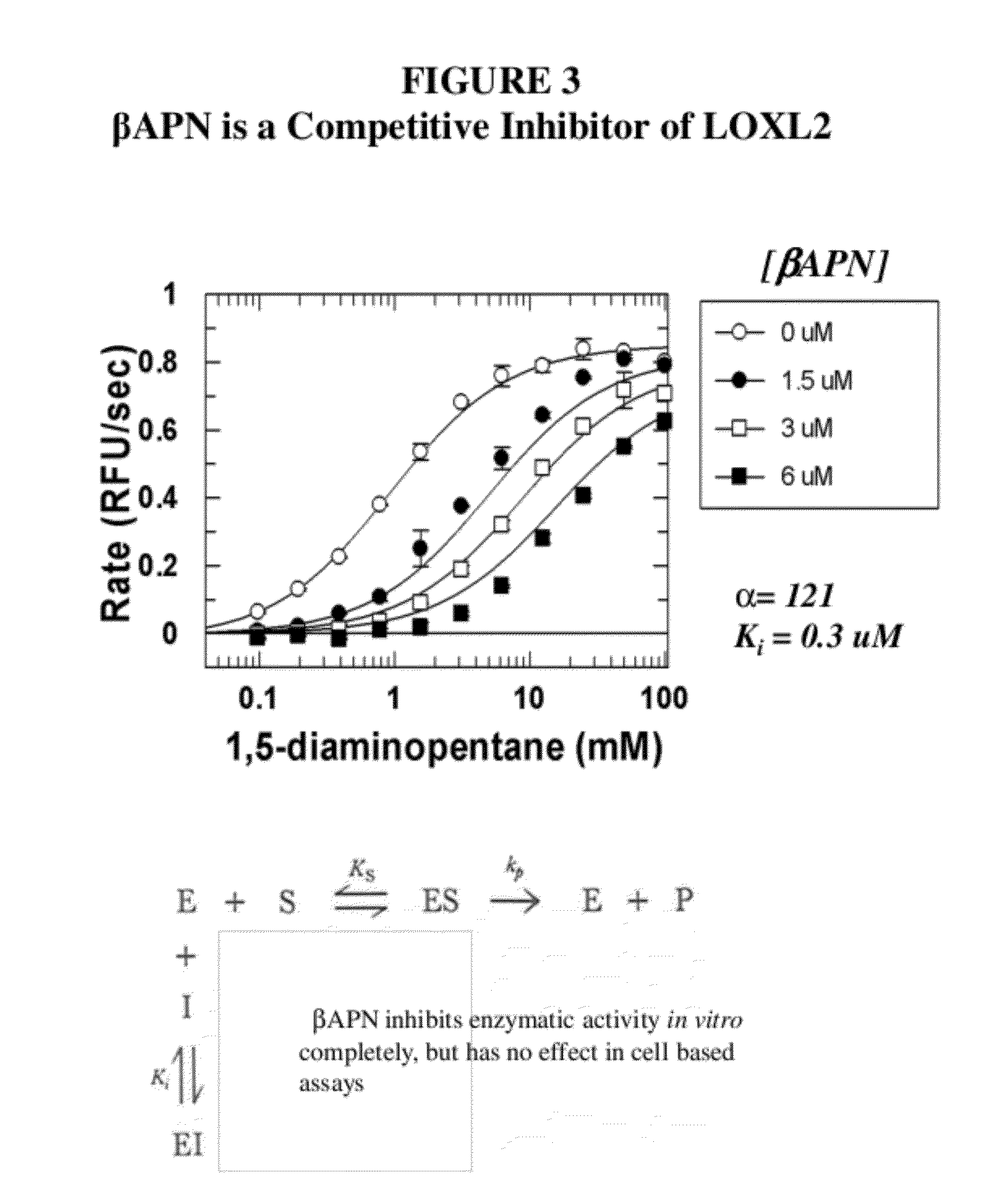
Lox and loxl2 inhibitors and uses thereof
a technology of loxl2 and inhibitors, which is applied in the direction of antibody medical ingredients, dna/rna fragmentation, fungi, etc., can solve the problems of cancer remains a major health problem, cancer the recurrence of detectable disease is a serious public health problem, so as to reduce tumor growth and reduce angiogenesis, the effect of inhibiting the enzymatic activity of lox
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Methods of Generating Murine Monoclonal Anti-LOX and Anti-LOXL2 Antibodies
[0559]Mice (BALB / c (00467)) were injected subcutaneously (s.c.), 5 times at 2-3 week intervals with 0.05 mg antigen (Ag) in an adjuvant formulation. For peptide Ags, peptides were conjugated to bovine serum albumin and formulated in Freunds Adjuvant (FA) prior to immunization. For protein Ags, the protein was formulated in Alhydrogel-Muramyl Dipeptide (ALD / MDP) adjuvant.
[0560]Mice were injected with Ag formulated in PBS, each day for 3 days via a combination of s.c., intraperitoneally (i.p.) and intravenous (i.v.) routes, 0.05 to 0.1 mg / route.
[0561]Cells from the spleen and lymph nodes of the mice were isolated and fused with P3X63-Ag8.653 myeloma cells using 50% polyethylene glycol.
[0562]Cells were cultured and a hybridoma library of HAT-selected cells was isolated essentially as described in Kenney, et al. (“Production of monoclonal antibodies using a secretion capture report web.” Biotechnology 13:787-790, ...
example 2
[0569]Anti-LOXL2 antibodies were screened using a protein screen B update to assess enzymatic activity of LOXL2.
[0570]Antibody candidates were initially chosen based on ELISA point tests. ELISA on multiple antigens was performed by Antibody Solutions and antibodies showing strong ELISA signal in the antigen of interest were selected for further characterization in enzymatic assays. LOXL2 produces hydrogen peroxide when the substrate 1,5-diaminopentane is deaminated and the enzyme regenerated.
[0571]Antibodies were assessed for their ability to inhibit enzymatic activity using a biochemical assay that couples the production of peroxide (liberated by LOXL2) to HRP and measuring the conversion of amplex red to a fluorescent product. Antibody hybridoma supernatant (10 μL) was added to 40 μL enzyme mixture (62.5 mM sodium borate pH 8.0, 5 units / mL HRP, 125 nM LOXL2, 10 ppm antifoam) and incubated at room temperature for 1 hour in a 96 well full area black plate. Enzymatic reaction was sta...
example 3
Anti-LOXL2 Antibody AB0023 and Enzymatic Activity
[0574]Enzymatic Activity of anti-LOXL2 antibodies can be assessed and IC50s determined.
[0575]M1, M1 (asc), M20 and M20 (asc) were assessed in the presence of 25 nM LOXL2 and 15 mM 1.5 DAP over increasing concentrations of antibody.
IC50 Determinations
[0576]Dose responses on selected antibodies were carried out against LOXL2 using the coupled enzymatic assay described above. A dilution series of antibody was created in PBST (0.01% tween-20) and 10 μL of this was added to 40 μL of enzyme mixture (62.5 mM sodium borate pH 8.0, 5 units / mL HRP, 125 nM LOXL2, 10 ppm antifoam) and incubated at room temperature for 1 hour in a 96 well full area black plate. Enzymatic reaction was started with the addition of 50 μL of substrate solution (50 mM sodium borate, 100 μM amplex red reagent, 20 mM 1,5-diaminopentane, 10 ppm antifoam) and read in an M5 plate reader using conditions described above. The slopes of the fluorescence response as a function ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com