Method for the immobilization of cationic active ingredients on surfaces
a technology immobilization methods, applied in the field of immobilization of cationic active ingredients on surfaces, can solve the problems of high complexity and cost, deactivation of cationic antimicrobial active ingredients, etc., and achieve the effect of preventing the surface from sliding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Testing the Effect of the Antimicrobial Finishing
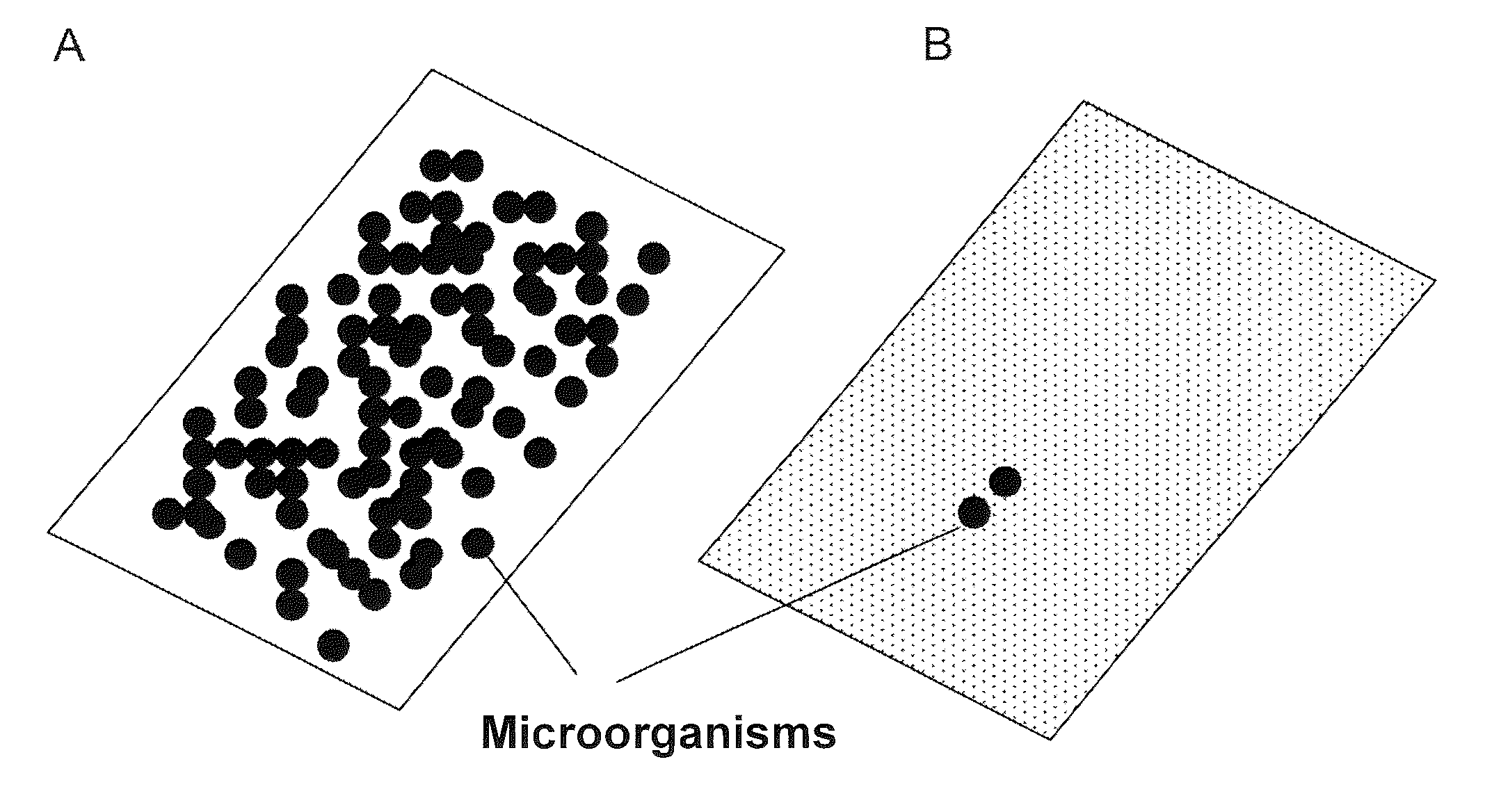
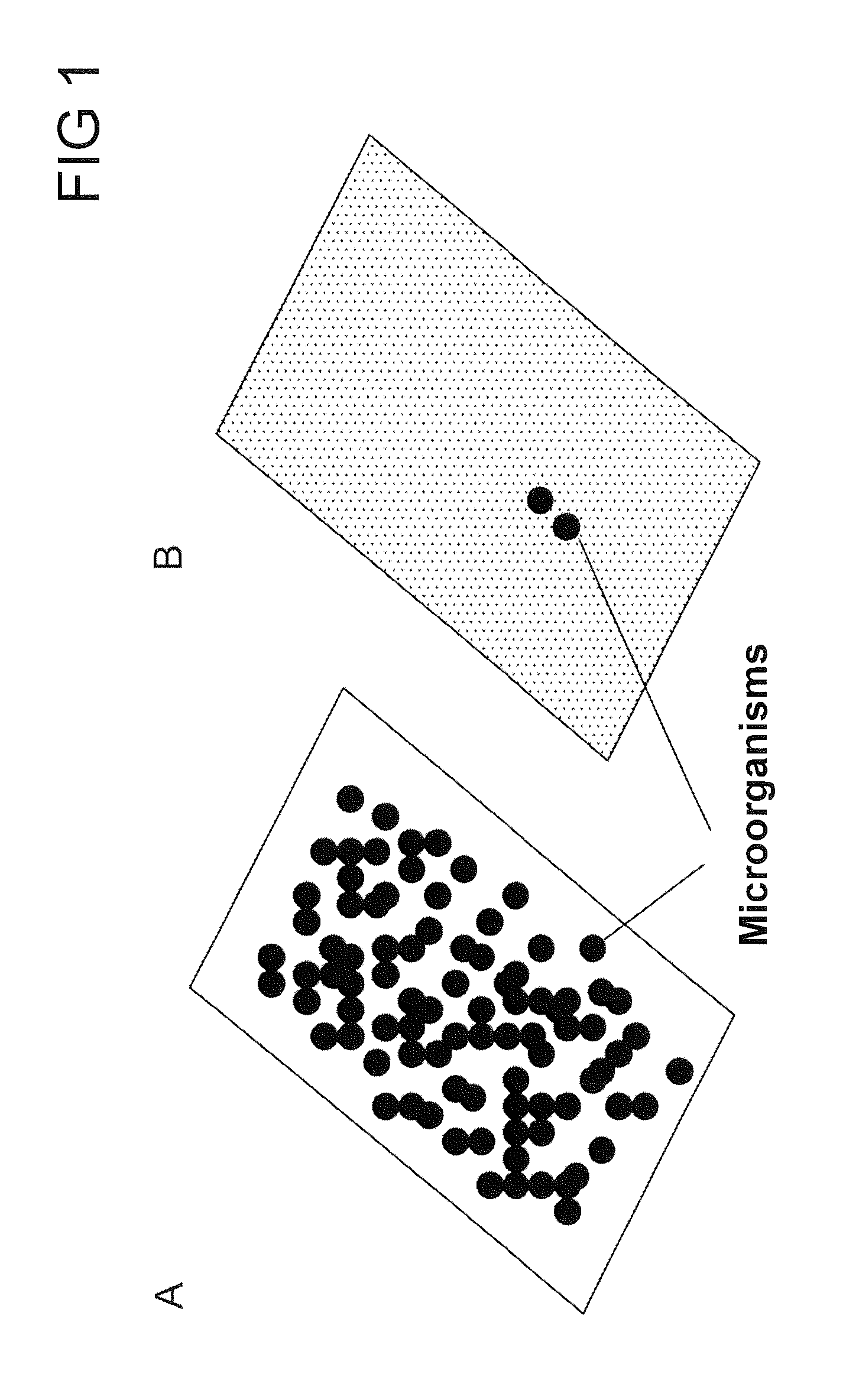
[0266]The effect of the antimicrobial finishing of the investigated surfaces was determined by means of a statistical biofilm assay. For this, the surfaces to be tested were cut into round disks which fit into the holes of 24-well microtiter plates. In order to prevent the surfaces from slipping during the assay, the surfaces were immobilized on the bottom of the microtiter plates with the help of glass joint grease.
[0267]The formation of biofilms was carried out starting from a preculture. For this, 20 ml of the standard commercial TSBY medium (Difco Laboratories, MI, USA) were inoculated with the help of a steady-state overnight culture of Staphylococcus epidermidis DSM1798, Escherichia coli DSM 5698, Proteus mirabilis DSM 4479 or Escherichia coli BL21 (DE3) with an optical density of 0.1. The preculture was incubated for 2 to 4 hours with agitation (at 200 rpm) and 37° C. until an optical density of from 2 to 3 was reached. In orde...
example 2
Antimicrobial Finishing of Silicone through Adsorption of PHMB
[0269]Prior to the adsorption, the silicone surfaces (disks with a diameter of 15 mm suitable for 24 well microtiter plates) were washed twice with the help of an SDS solution (10 mg / ml in ultra-pure water). Rinsing was then carried out twice with ultra-pure water and the surfaces were degreased by dipping into ethanol (70% in ultra-pure water), sterilized and then dried.
[0270]The adsorption of the antimicrobial active ingredient (W), here of the active ingredient polyhexamethylenebiguanide (also PHMB or Reputex 20 as 20% solution) was carried out following dilution to 50 mg / ml in PBS (phosphate-based saline solution). The silicone surfaces to be coated were incubated with the Reputex 20 solution for 1 hour at 400 rpm on the Eppendorf shaker. The surfaces were then dried.
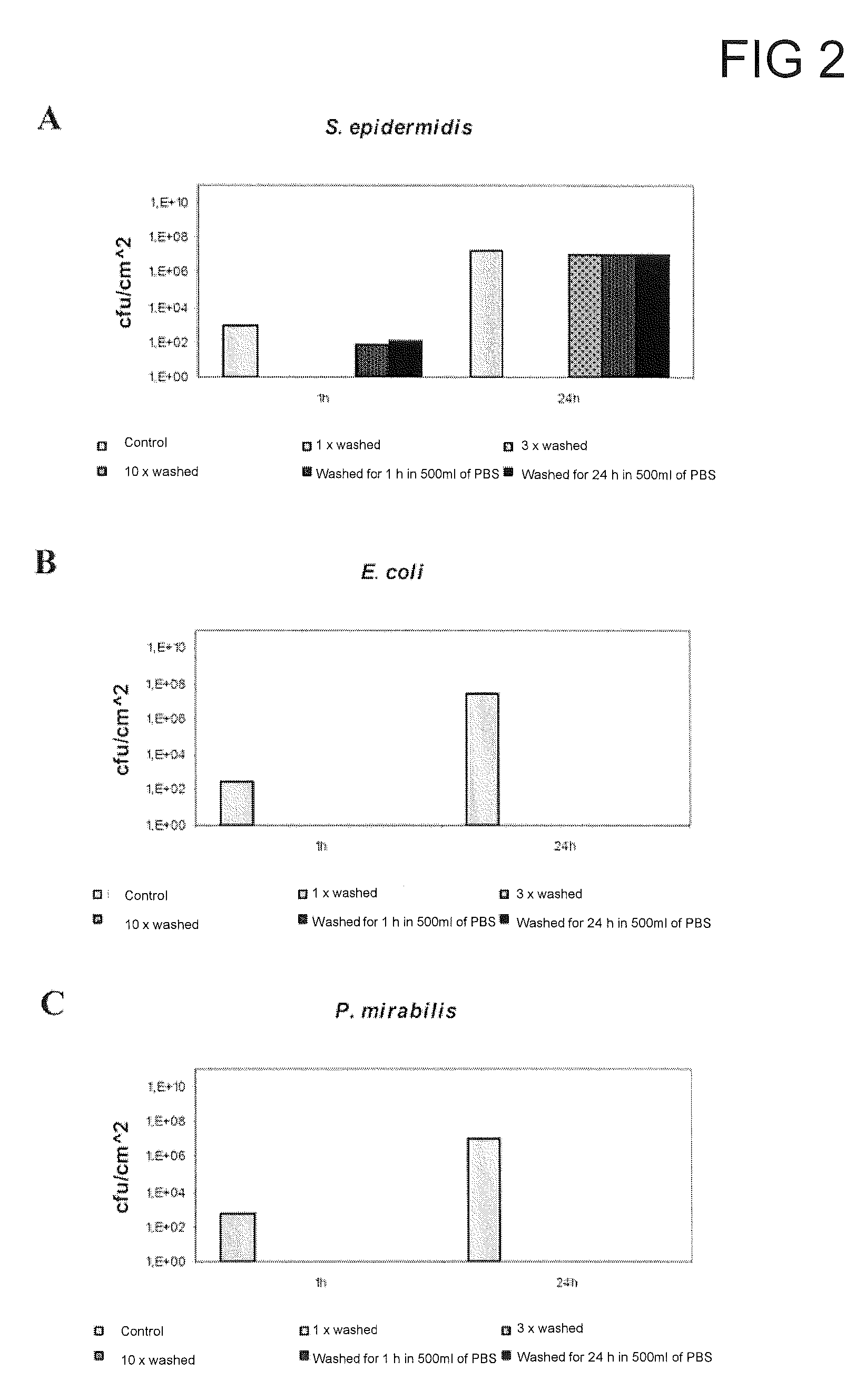
[0271]In order to test how durably the cationic antimicrobial active ingredient has been bonded to the surface, the surfaces were washed prior to the bio...
example 3
Antimicrobial Finishing of Silicone Surfaces by Adsorption of Polyhexamethylenebiguanide with the Help of Hydrophobin A in the Two-Step Method
[0278]The antimicrobial finishing of the silicone surfaces was carried out in two steps. In the first step the hydrophobin A was bonded to silicone as hydrophobin component (H). For this purpose, the surfaces were incubated with 0.5 mg / ml of hydrophobin A in binding buffer (50 mM Tri-HCl, 1 mM CaCl2 at pH 8.0) for at least 3 hours. Preferably, the hydrophobin yaad-Xa-dewA-his (SEQ ID NO: 20) (see WO 2006 / 082251) is used here. Washing is then carried out twice with ultra-pure water and the surface is dried.
[0279]In the second step, polyhexamethylenebiguanide (Reputex 20) was absorbed onto the hydrophobin coating. For this purpose, the surfaces were overlaid in each case with 1 ml of 50 mg / ml Reputex 20 in PBS and incubated for one hour at room temperature. Washing was then carried out once with 1 ml of ultra-pure water.
[0280]In order to test ho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com