Compositions Containing Berberine or Analogs Thereof for Treating Rosacea or Red Face Related Skin Disorders
a technology of rosacea and red face, applied in the direction of drug compositions, immunological disorders, antibacterial agents, etc., can solve the problems of unfavorable treatment of rosacea by other anti-inflammatory agents and unfavorable treatment of rosacea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
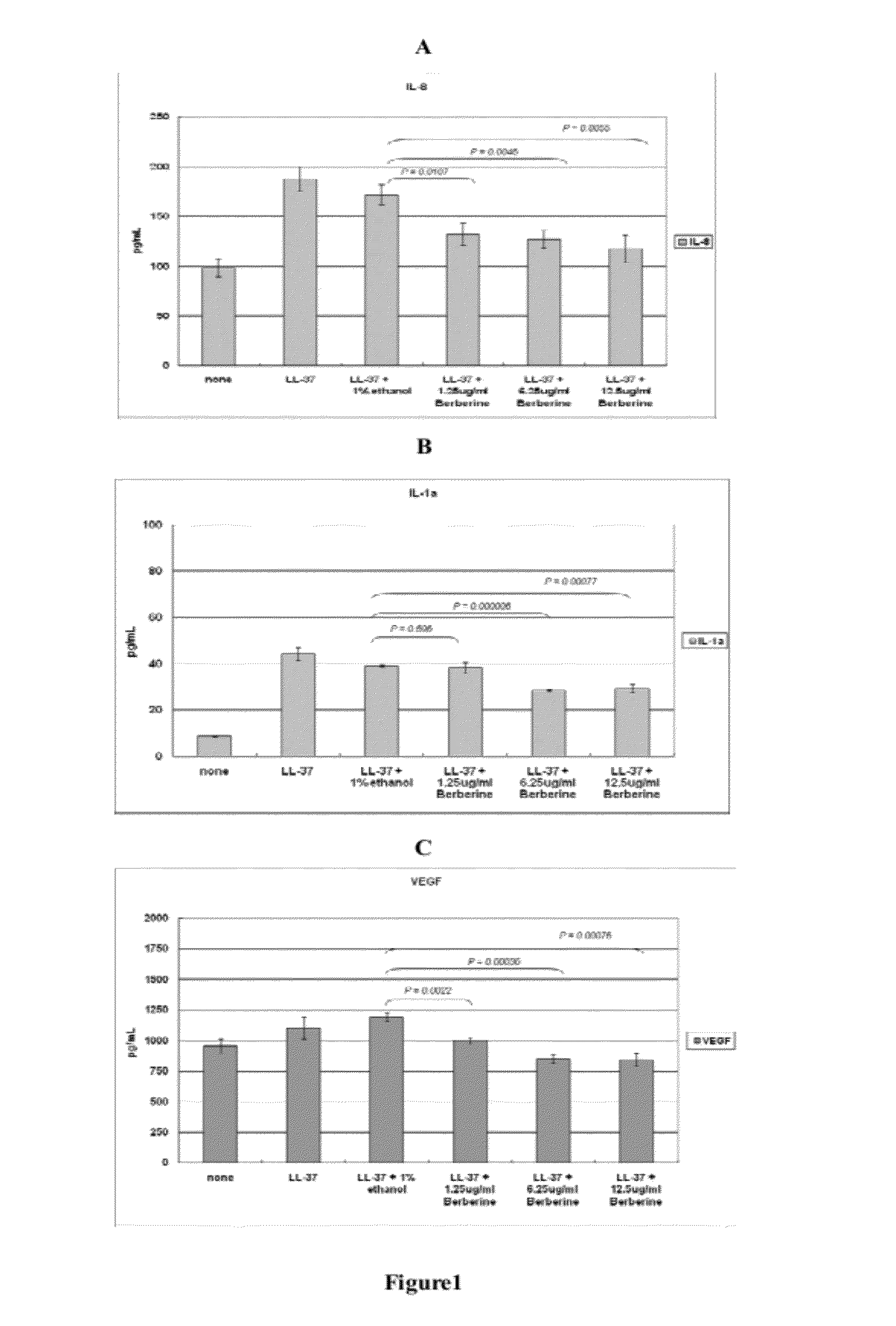
Effects of Berberine on Inhibiting Cathelicdin Peptides-Induced Cytokine Secretion by Human Keratinocytes (In Vitro Assay)
[0028]For our in vitro study, berberine (Sigma, St. Louis, Mo., USA) was dissolved in water, methanol, ethanol or dimethyl sulfoxide (DMSO). Normal human keratinocytes (Invitrogen, CA, USA) were grown in EpiLife medium (Invitrogen, CA, USA) supplemented with 0.06 mM Ca+2, 1% EpiLife defined growth supplement, and 1% penicillin / streptomycin (Invitrogen, CA, USA). Cells were grown at 37° C. in a humidified atmosphere of 5% CO2 and 95% air. The human keratinocytes were cultured to confluence and treated with synthetic cathelicidin peptides (LL-37) (6.4 μM) for 16 h to induce inflammatory response similar to that observed in rosacea. Some of the cathelicidin-treated keratinocyte cultures were co-incubated with berberine of concentrations from 1.25 μg / ml to 12.5 μg / ml. The keratinocytes cultures treated with cathrlicidin or cathrlicidin with 1% ethanol and without ber...
example 2
Preparation of Topical Pharmaceutical Formulations Containing Purified Berberine and Palmatine at Defined Percentages
[0030]Based on the rationale described above, the topical berberine-containing pharmaceutical formulations of this invention have one key feature: it contains purified berberine at defined percentages that are higher than can be obtained in previous formulations using extracts of berberine-rich plants. The ranges of concentrations were subjected to tests in animal model studies and human clinical studies.
[0031]For our studies on animal models and human patients, purified berberine was dissolved in 100% ethanol, and then water was added to reach a desired concentration of berberine in the final solution. In the gel formulation, for example, 0.1% or 0.2% berberine was prepared in 10% ethanol. The solution or gel formulation were capped and stored at 4° C. until use. The results of our studies in animal models and human patients with rosacea indicate that the concentrati...
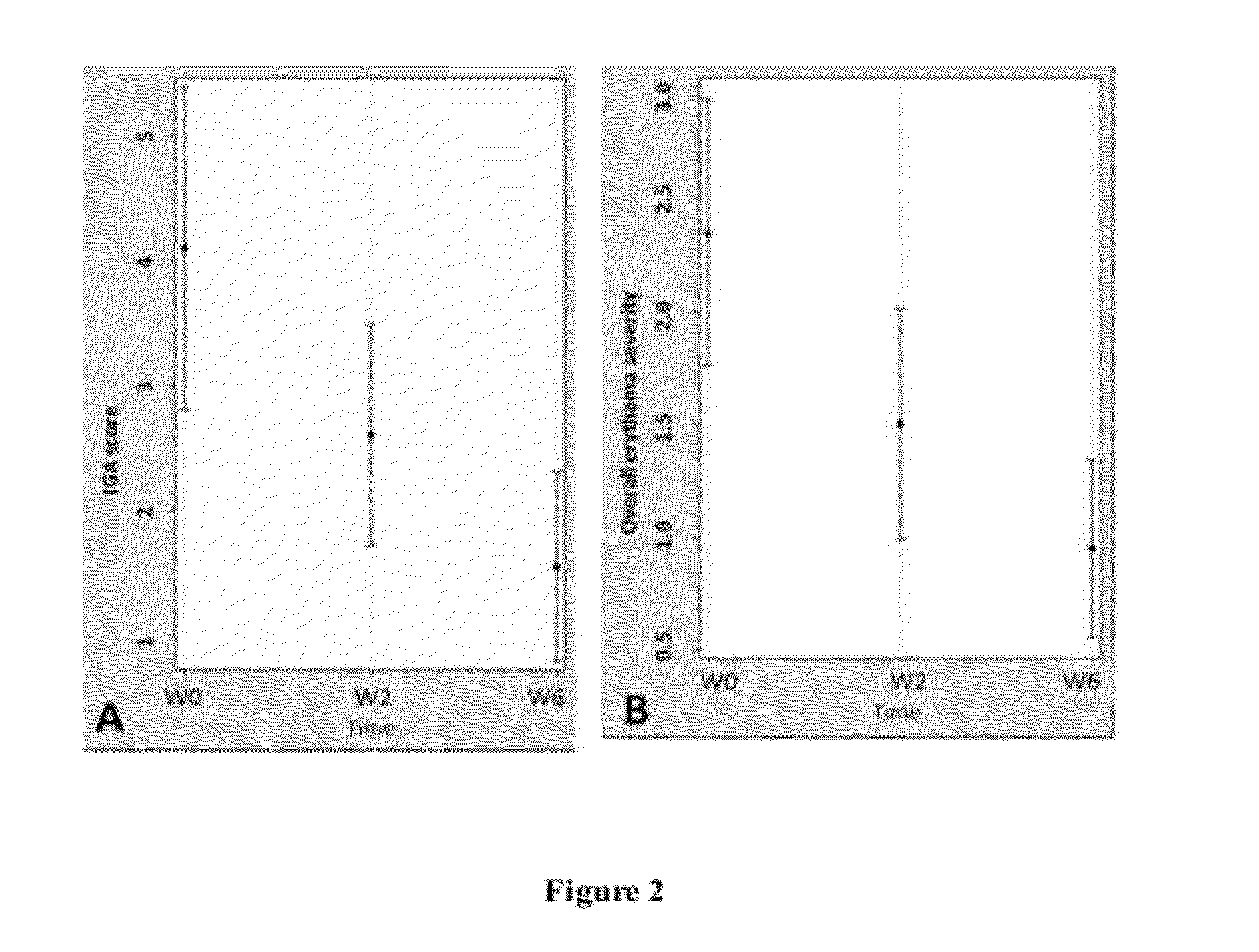
example 3
The Effects of the Topical Pharmaceutical Preparation of this Invention on a Mouse Model of Rosacea
[0035]The animal model of rosacea: the animal model of rosacea was adopted from previous reported18. Briefly, BALB / c and C57BL / 6 mice, shaved 24 h before treatments, were injected subcutaneously on the back with 40 μl of cathelicdin peptide (320 μM) twice a day. Forty-eight hours after the initial injection (four injections in total), erythema and edema were observed on the injected site mimicking the clinical features of rosacea.
[0036]In our experiments, cathelicidin-injected mice were treated with or without topical berberine twice a day to observe the effect of berberine on reducing inflammation. The results showed that mice given subcutaneous injections of cathelicidin peptides induced erythema and vascular dilatation in the skin, which resembled clinical features of rosacea after 48 h. The cathelicidin-injected mice were then divided into 2 groups, which were treated with berberin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com