Fucoidans as Ligands for the Diagnosis of Degenerative Pathologies
a degenerative pathology and ligand technology, applied in the field of ligands for the diagnosis of degenerative pathologies, can solve the problems of complex preparation and purification of antibody-based imaging agents, preventing industrial development and commercialization, and achieving high affinity, specificity and/or selectivity for selectins, excellent sensitivity, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
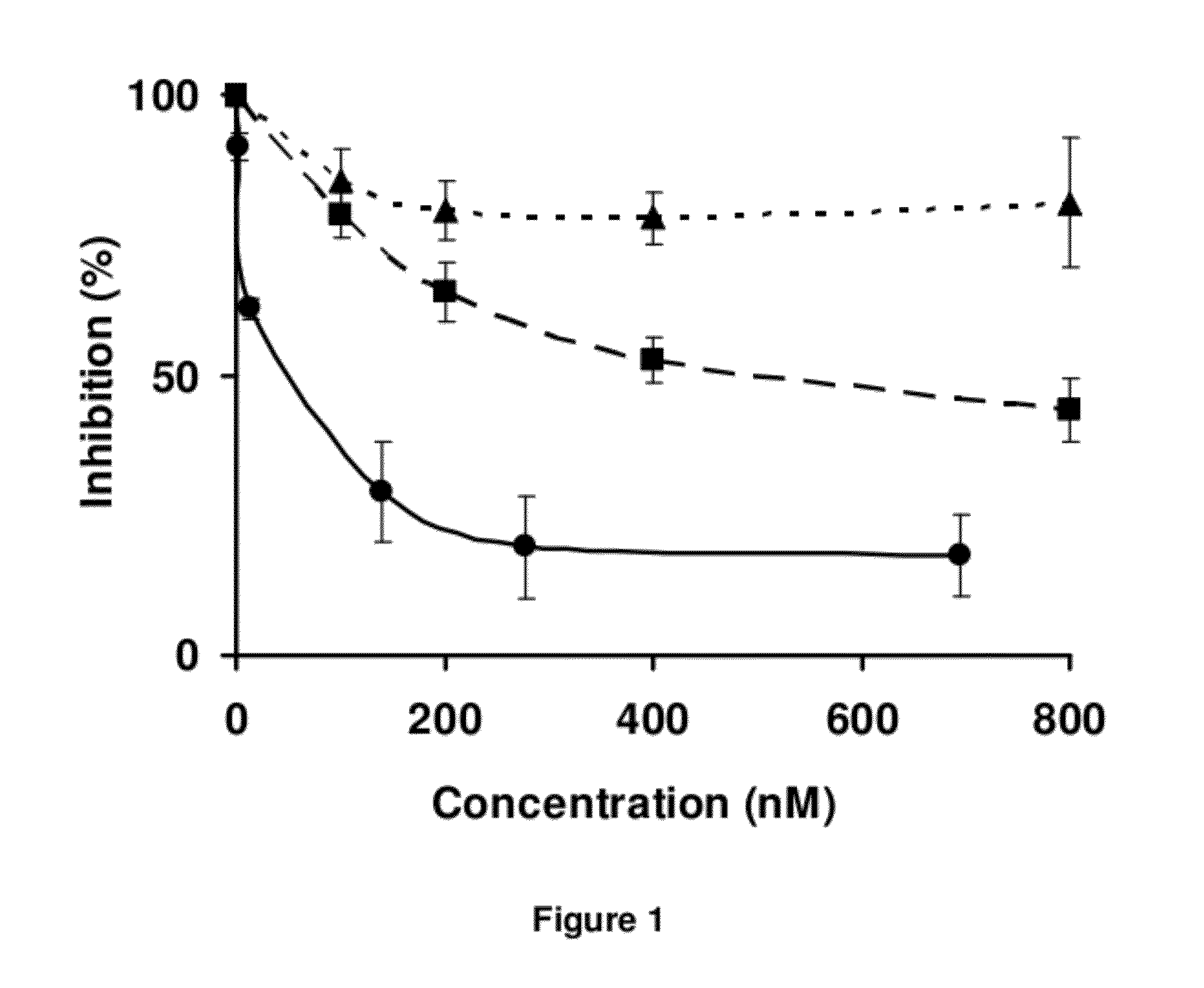
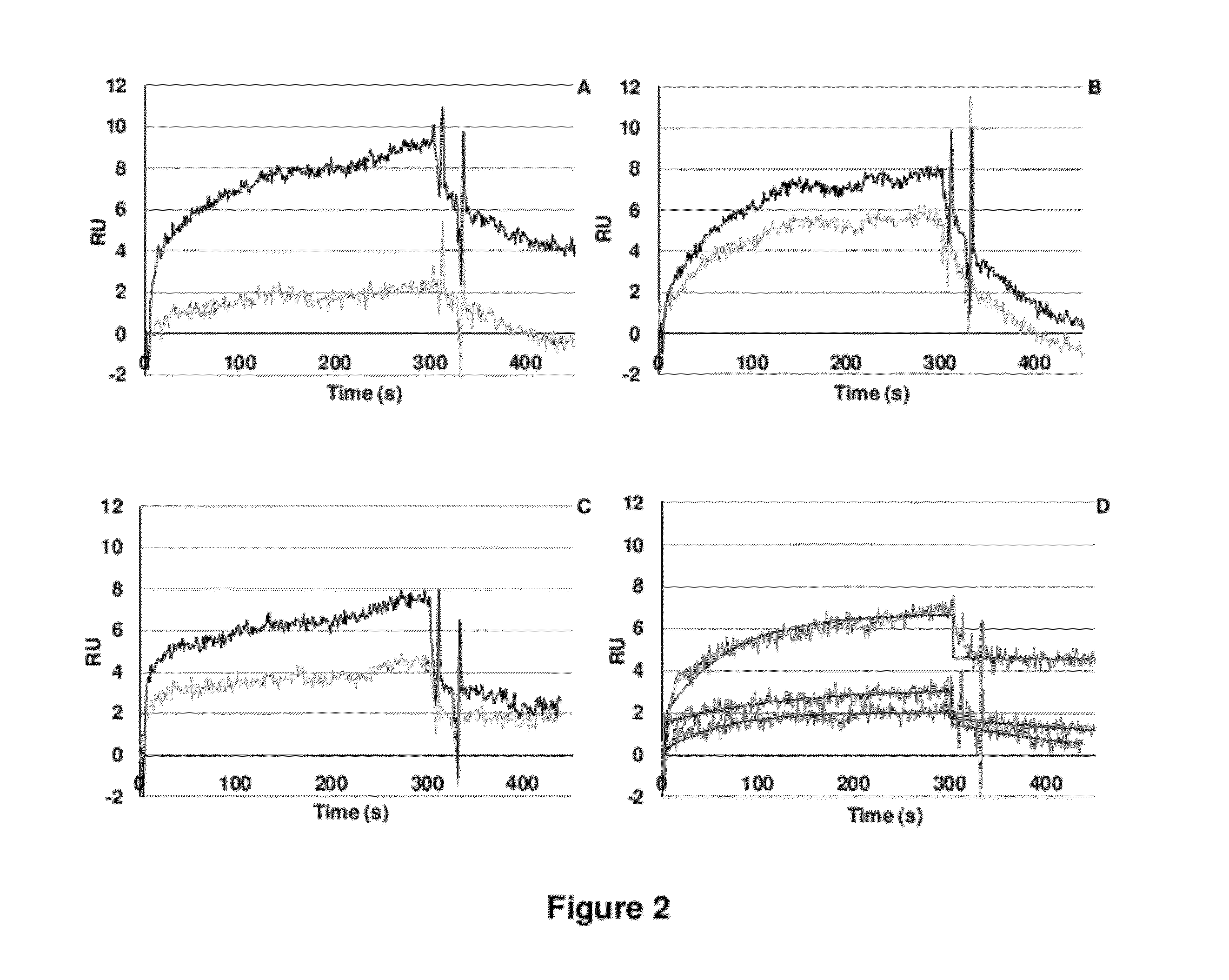
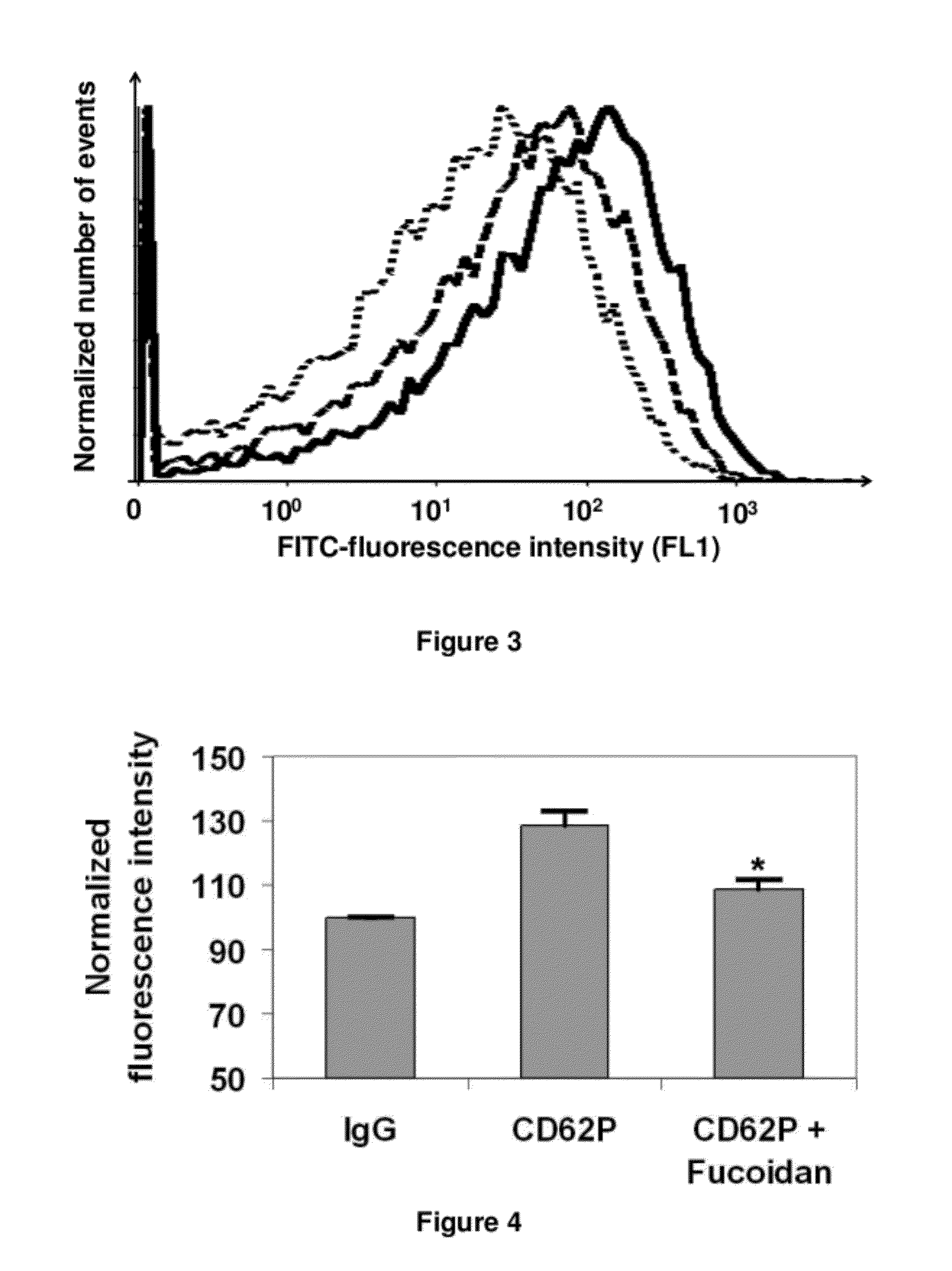
LMW Fucoidans are Highly Specific Ligands of P-Selectin
Materials and Methods
[0125]Chemical Products. Fluorescein isothiocyanate (FITC) was purchased from Fluka (Saint-Quentin Fallavier, France); streptavidin-peroxidase conjugate from Dako (Trappes, France); diaminopropane, sodium cyanobromohydride and peroxidase substrate ABTS from Sigma-Aldrich (Saint-Quentin Fallavier, France); sinapinic acid solution from Bio-Rad Laboratories (Hercules, Calif., USA); and the amine coupling kit and running buffer from BIAcore (Uppsala, Sweden).
[0126]Polysaccharides. The low molecular weight fucoidan (based on sulfated repeating fucose unit; M=7200 g / M; SO4=30% (w / w)) was prepared from brown seaweed as previously described (A. Nardella et al., Carbohydr. Res., 1996, 289: 201-208). The low molecular weight heparin (M=5700 g / M; SO4=45% (w / w)) and low molecular weight dextran sulfate (M=8000 g / M; SO4=52% (w / w)), were supplied from Sigma-Aldrich; and the biotinylated polyacrylamide-type glycoconjugate ...
example 2
99mTc-labelled Fucoidan as P-selectin-targeted Imaging Agent for the in vivo Scintigraphic Detection of Platelet Activation and Accumulation
[0147]Fucoidan was labelled with technetium-99m (99mTc) using the well-known stannous reaction in solution. Briefly, 4 μL of stannous chloride were added to 10 μL of fucoidan (1 mg / mL, MW=7200) followed by 2 μL of potassium borohydride. Immediately after combination of these reagents, 50 μL of 99mTc (corresponding to 15-30 mCi) were gently added to the mixture. The labelling reaction was complete after 1 hour of incubation. Control of the labelling was performed using thin layer paper chromatography and methyl-ketone as eluant. The percentage of labelling was 100%.
[0148]Rat models of endocarditic vegetations, aneurysmal and atrial trombi were used as animal models of clinical conditions associated with platelet activation and fibrin formation. Intravenous injection of 1 μg of 99mTc-labelled fucoidan allowed the in vivo visualization of platelet-...
example 3
Preparation of fucoidan-coated USPIO Particles
[0150]The present Applicants have developed five different strategies to coat fucoidan onto USPIO particles.
[0151]The first strategy involves the synthesis of iron particles in the presence of unmodified fucoidan. The Applicants have applied a method of synthesis previously described with dextran (R. S. Molday et al., J. Immunol. Methods, 1982, 52: 353-367) replacing dextran MW 40000 by fucoidan MW 50500. Fucoidan-coated iron nanoparticles were obtained. However, these particles were found to be unstable in water.
[0152]The second strategy comprises the coating of an acidic ferrofluid with unmodified fucoidan. Fucoidan was incubated with acidic ferrofluid. Fucoidan-coated iron nanoparticles were obtained that were stable in aqueous medium pH 7.4, but unstable in buffers with ionic strength of 0.15 M, which are used in most applications. The synthesis could be obtained in the presence of a cross-linker (A. San Juan et al., J. Biomed. Mater...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dissociation constant | aaaaa | aaaaa |
| dissociation constant | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com