Non-invasive diagnostic method for the evaluation of intestinal lactase deficiency (hypolactasia)
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0079]Two groups of subjects were selected. A first group composed of 42 healthy controls and a second group composed of 205 subjects with a clinical history suggestive of lactose intolerance. Firstly, the lowest suitable oral dose of 4-GX administered to healthy volunteers was determined that enabled reliable detection (in terms of accuracy and reproducibility) of xylose levels in urine and blood via the analytical method to be used. In addition, the tolerance of these subjects to the various doses of 4-GX used in the test and the pharmacokinetics for each dose administered were analysed, both in urine and blood. The doses of 4-GX used were: 0.125 g, 0.250 g, 0.5 g, 1 g, 3 g and 6 g in addition to placebo in 12 healthy volunteers with washout period between doses of between three and seven days. It should be appreciated that the 4-GX doses can include small quantities of water which remain subsequent to the drying process of the 4-GX product. For example, the 4-GX p...
example 2
Selection of the Optimum Dose and Time for the Diagnosis of Lactose Intolerance by the Oral Administration of 4-GX
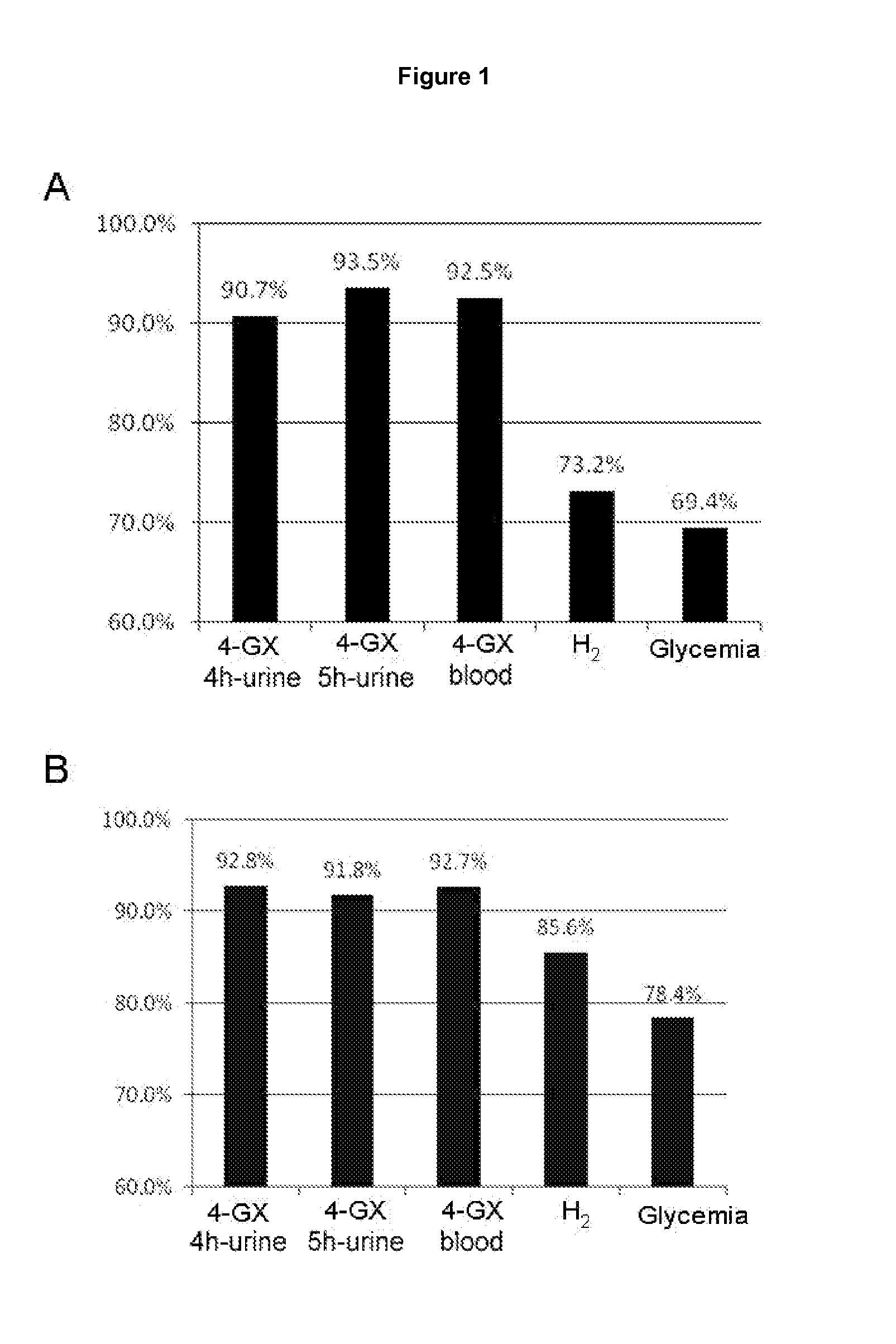
[0088]The main pharmacokinetic parameters that were evaluated for urine excretion of xylose were the maximum rate of urinary excretion (U rate max) and the total quantity of xylose excreted over the period of observation (Ae 0-t); using the amounts of xylose excreted in each urine sample collection period, the quantities accumulated for each interval were calculated so that it was possible to determine the minimum urine collection time that would distinguish accumulated excretion at the various doses compared to placebo (Table 1).
[0089]The statistical analysis of the pharmacokinetic parameters of urinary xylose excretion showed that all the doses of 4-GX, in terms of the maximum urinary excretion rate (U rate max) and accumulated excretion over 8 hours of duration of the observation period (Ae 0-t) were significantly different from that obtained with placebo. The analysi...
example 3
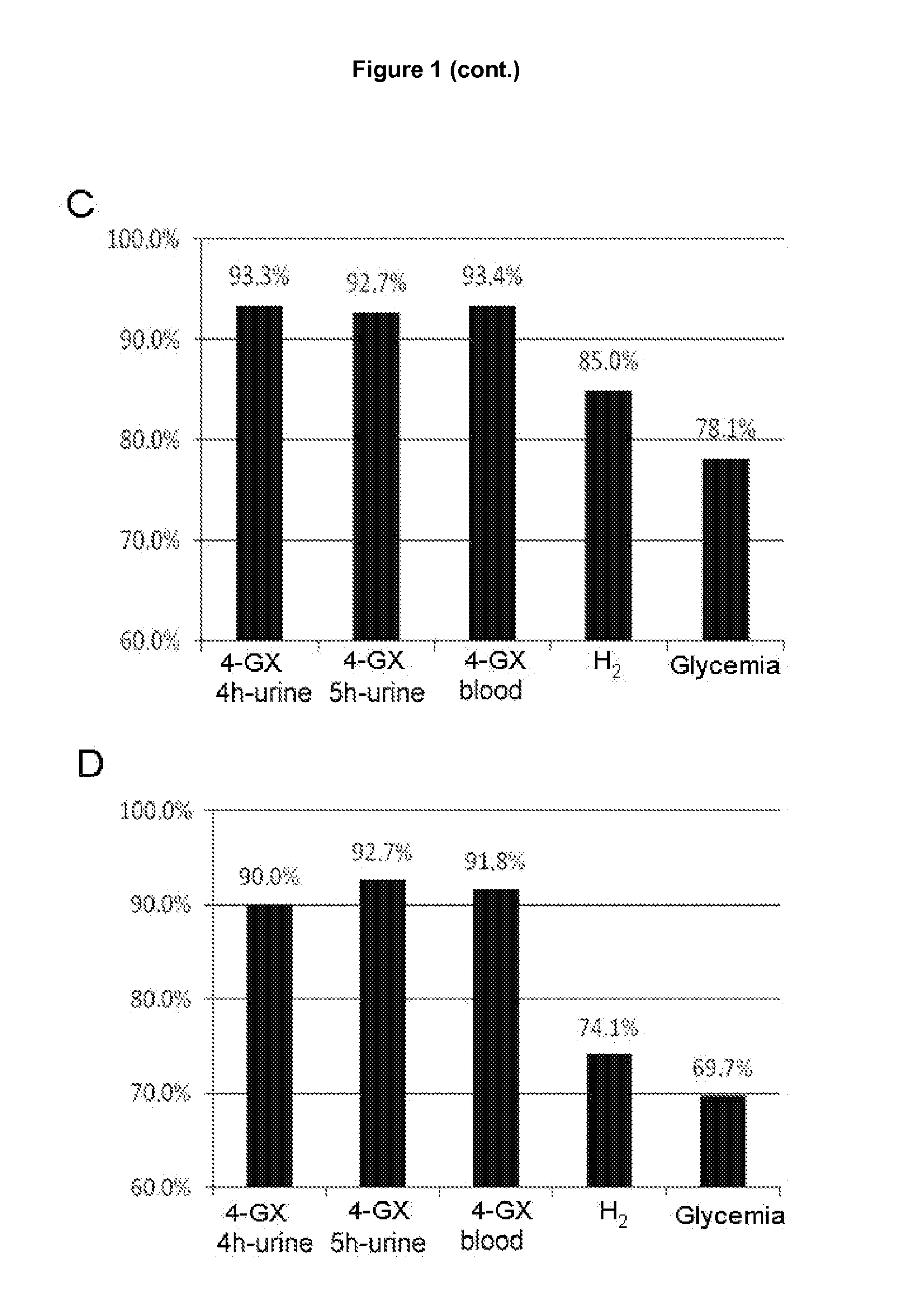
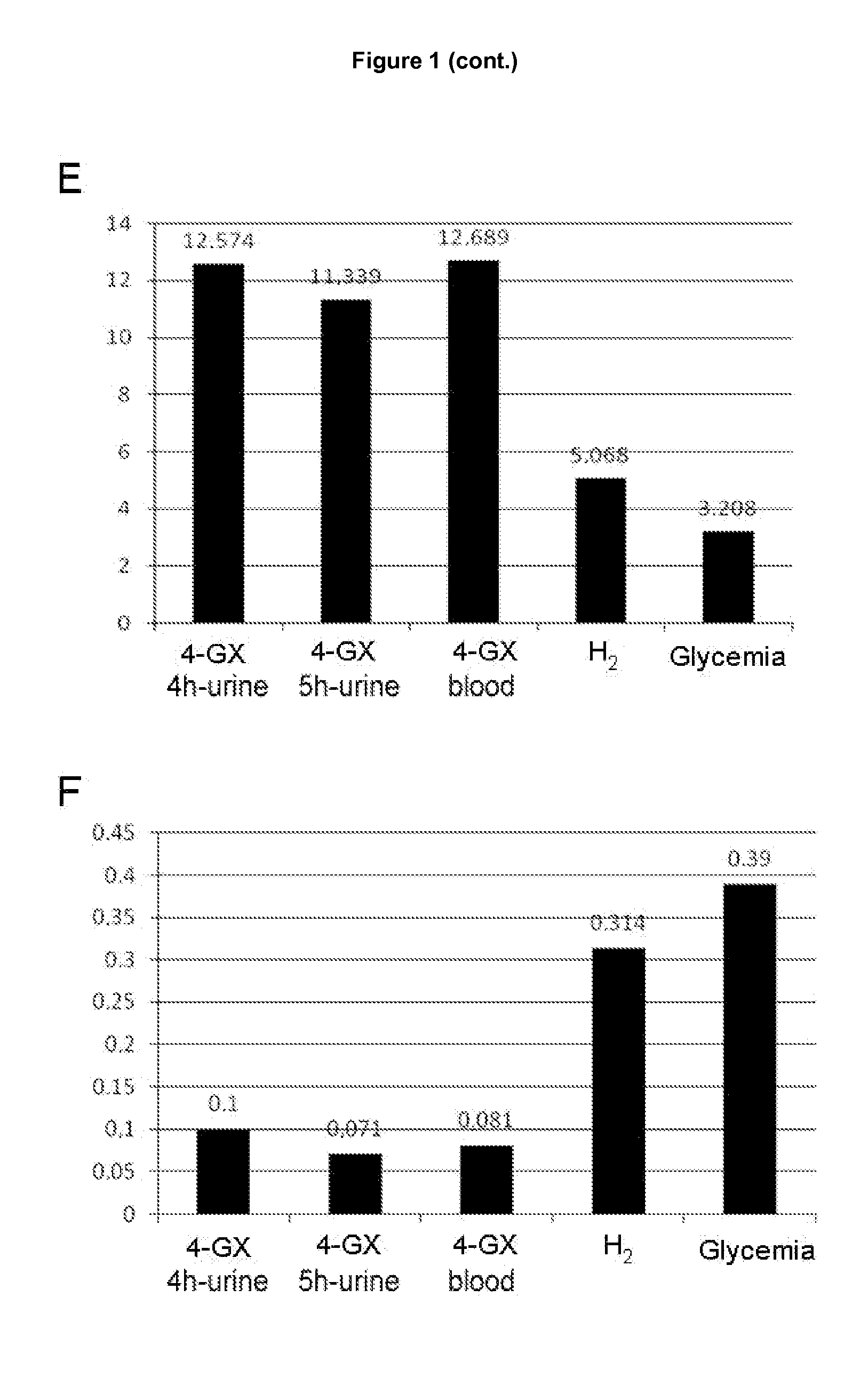
Determination of the Lower Limits of Normal of the Total Quantity of Xylose Excreted in Urine and Blood Concentration in Healthy Control Individuals
[0093]The data obtained with the 4-GX administration test in the healthy control group was used to determine the lower limits of normal of xylose in urine and blood (cut-off points). The cut-off points for the 4-GX test in urine were obtained for times of 4 hours and 5 hours because, as already seen in example 2, these times are the best for determining the amount of xylose excreted in urine following 4-GX administration and performing a reliable diagnosis of lactase deficiency. Similarly, the cut-off points for the determination of the blood xylose concentration following 4-GX administration were obtained for the time of 90 minutes following ingestion of the disaccharide. The difference between the average xylose concentration (in blood and the total quantity of xylose excreted in the urine) and 1.96 times the standard deviation was use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com