Methods and systems for the quantitative chemical speciation of heavy metals and other toxic pollutants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
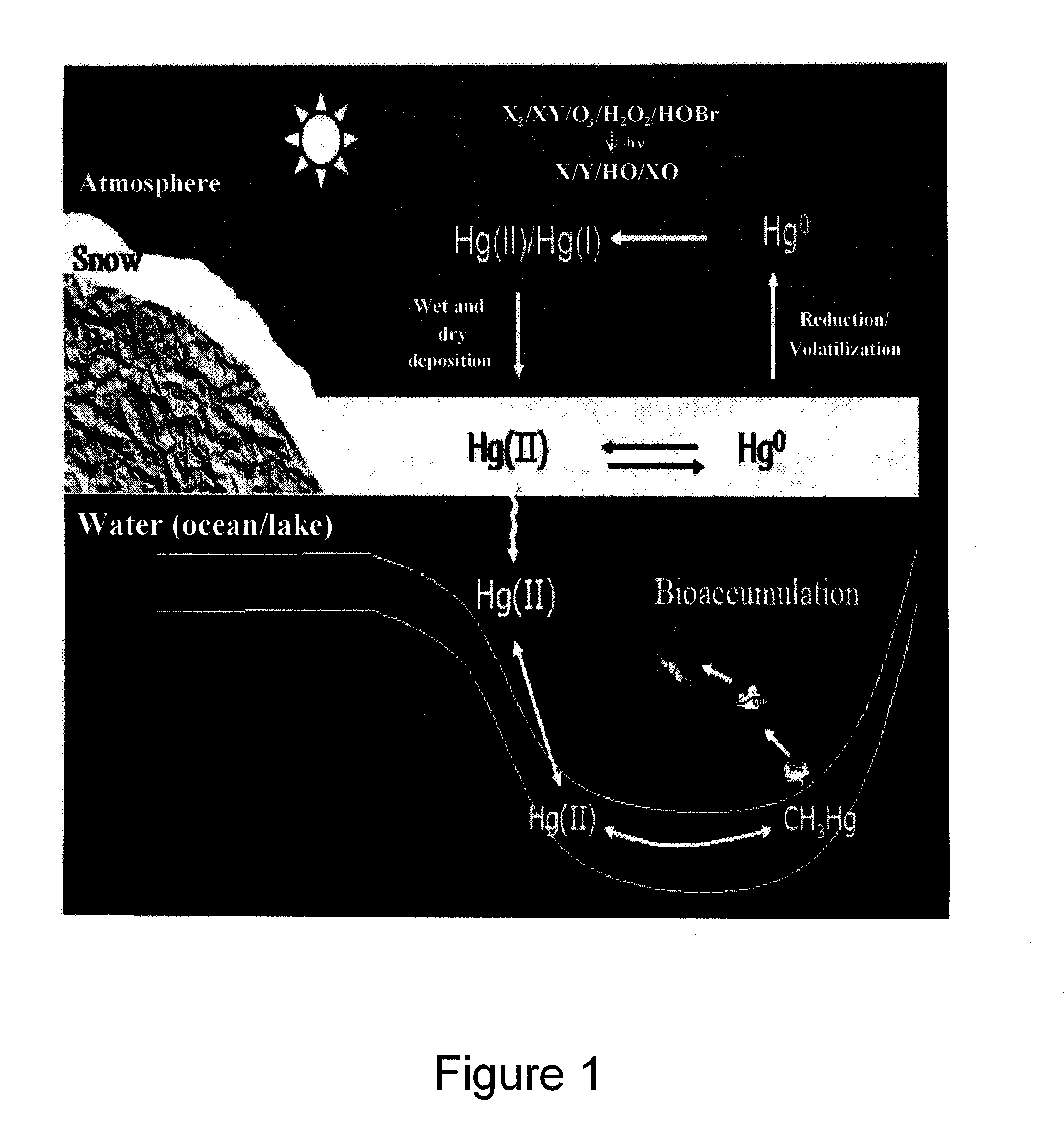
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the System
[0046]a. Preparation of Gold Nanoparticle-coated Fiber and Trap
[0047]Single layer Au-nanoparticle surfaces are coated onto stainless-steel interface and / or a wire for mercury capture. The process begins by cleaning the stainless-steel wire with a 3:1 mixture of concentrated H2SO4 and 30% H2O2, both to remove trace organics and other contaminants and to increase the number of pendant oxygen atoms available for silanization on the surface. The cleaned wire was then immersed for two minutes in a solution containing 60 μL of 3-(aminopropyl)-trimethoxysilane (APTMS) dissolved in 15 mL of a 3:1 mix of 18.2 MΩ water and ethanol. After silanization, loose silanes were removed from the surface by rinsing with ethanol, and the wire / filter / trap was blown-dry with UHP N2 gas. The APTMS was allowed to cure at room temperature for several hours before continuing the SPME preparation.
[0048]Once the APTMS-covered wire had cured, it was immersed in a gold nanoparticle colloi...
example 2
Analyte Collection for Sample
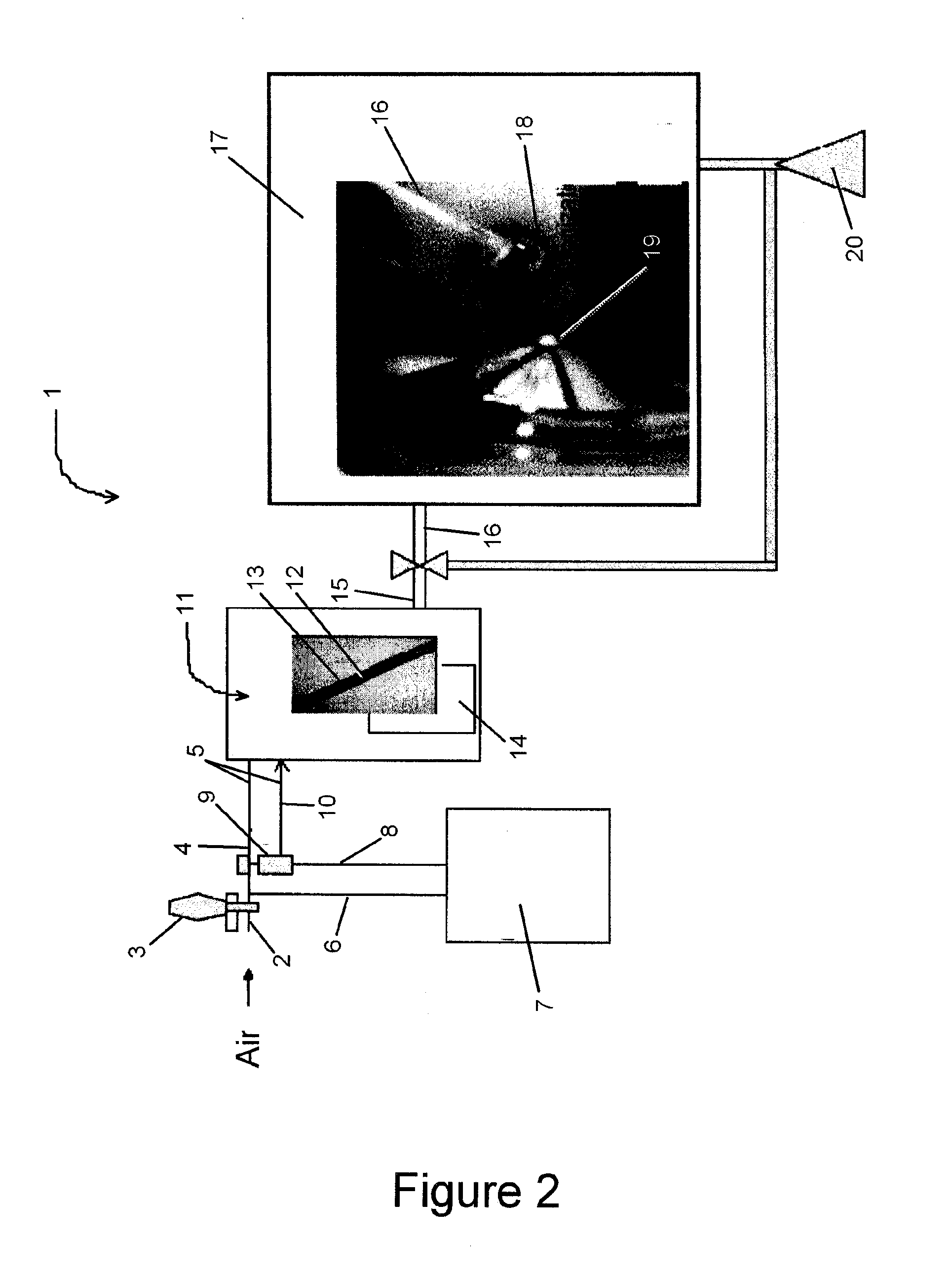
[0064]a. Example of Collection Using a Sampling Flask as an Interface
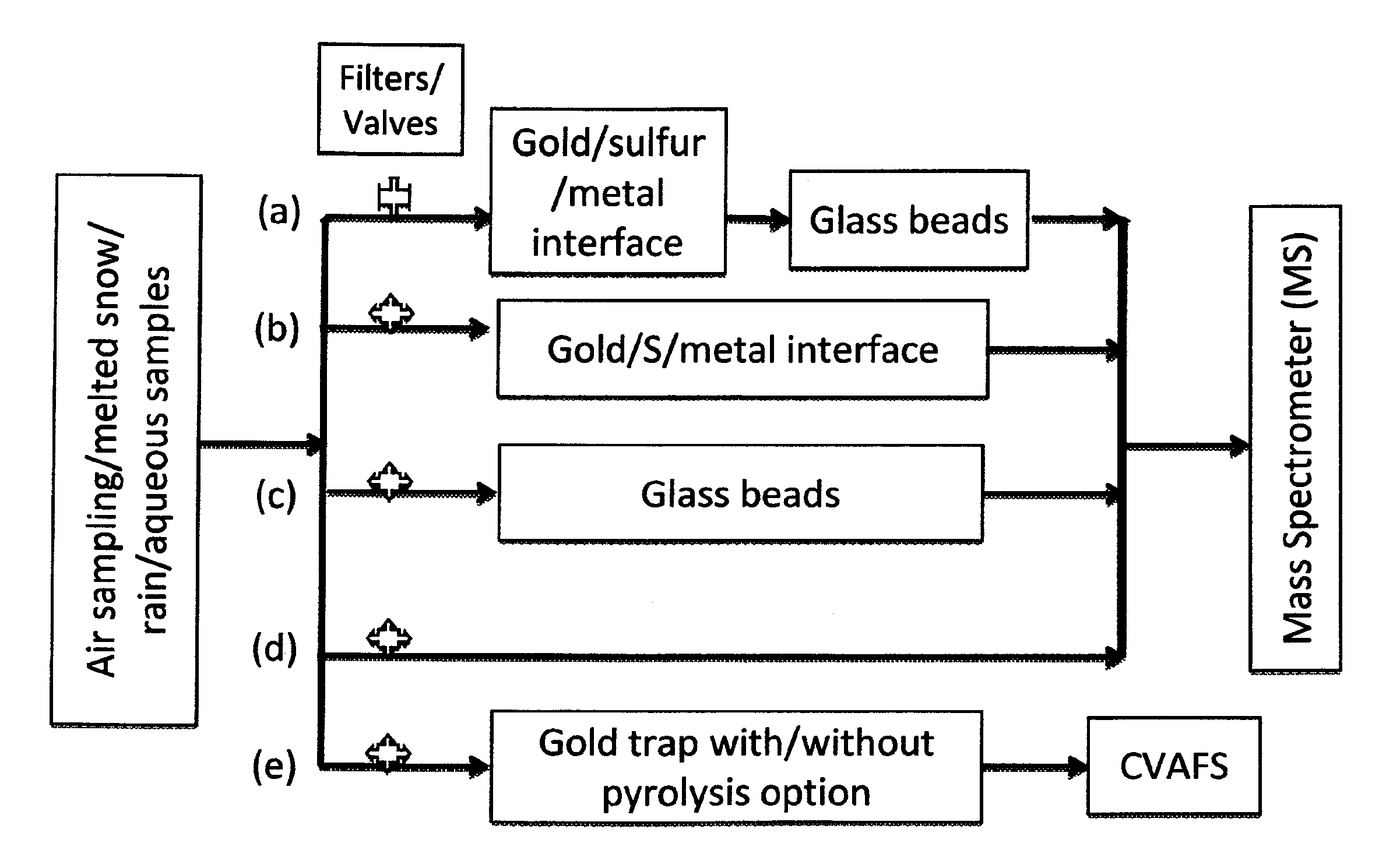
[0065]Gold and / or sulfur nanoparticle-coated surfaces (e.g. Fe / Au) were preconditioned for several minutes under vacuum at a temperature of ca. 360° C. before insertion into a ˜2 L air sampling flask. The sampling flask, as well as all FEP tubing up-flow of the flask, was washed several times with 1M nitric acid and 18.2 MΩ water. Air was passed through the air sampling flask at ca. 18 L per minute for a total time of 14-19 hours. In one extraction, a 0.45 μm Teflon™ filter was attached at the sample line inlet to prevent particulate mercury from entering the sample line.
[0066]b. Analyte Pre-concentration from Air
[0067]A series of physical and physicochemical traps for the collection of measureable quantities of oxidized mercury from large volumes of air have been developed. For example, traps included pieces of 10 cm long 6 mm diameter glass tubing containing gold-microparticle-coated ...
example 3
Calibration and Analysis
[0071]a. APCI-MS Analysis of Mercury Species Extracted from Air
[0072]Mercuric halides collected onto gold nanoparticles-coated fibers and traps were desorbed directly into the source of an atmospheric pressure chemical ionization mass spectrometer (APCI-MS) for detection. APCI-MS analysis of mercury halides is performed in negative mode (i.e. detection of negative ions only). The APCI-MS inlet accommodates both the fiber and a N2 carrier gas that flows around the outer tubing of the fiber at a rate of 0.3-3 L / min. Initially, the inlet is kept isothermal at 50° C. while the fiber is exposed to the gas stream. No gases are observed to desorb from the fiber / trap at this temperature. When the instrument baseline is stable, the inlet is ramped to 360-375° C. over the course of several seconds. The HgCl2 and HgBr2-gold amalgams destabilize in the temperature range of 300-330° C., resulting in a peak whose area can be integrated for quantification similar to the chr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com