Anti-thrombotic compounds
a technology of antithrombotic compounds and compounds, applied in the field of antithrombotic compounds, can solve the problems of reducing blood flow, affecting the treatment effect, affecting the survival rate of patients, etc., and achieves the effects of preventing side effects, preventing bleeding, and avoiding side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of mixture of (7aS,2′S) / (7aR,2′S)-isomers of 2-oxoclopidogrel
a) Methyl-(R)-2-hydroxy-2-(2-chlorophenyl)acetate
[0082]In a four necked round bottomed flask, 500 gm of (R)-2-chloromandelic acid was taken in 2000 ml methanol. Then 18.8 gm of sulfuric acid was added and heated to reflux, till completion of reaction. Then excess of methanol was distilled off under reduced pressure. Residue was taken in dichloromethane and washed with aqueous sodium bicarbonate solution. Dichloromethane was distilled under reduced pressure to obtain 522 gm of Methyl-(R)-2-hydroxy-2-(2-chlorophenyl)acetate as an oil. Yield: 94%. Purity:98.5%
b) Methyl(R)-2-(4-nitrophenylsulfonyloxy)-2(2-chlorophenyl)acetate
[0083]
[0084]In a four necked round bottomed flask, under nitrogen atmosphere, 640 ml of dichloromethane, 221 gm of 4-Nitro benzene sulfonyl chloride, 12.1 gm of 4-dimethylaminopyridine, and 200 gm of Methyl(R)-2-hydroxy-2(2-chlorophenyl)acetate were added. It was cooled to around 0° C., and 101...
example 2
Methyl (7aS,2′S)-2-(2-chlorophenyl)-2-(2,4,5,6,7,7a-hexahydro thieno[3,2-c]-5-pyridin-2-one)acetate
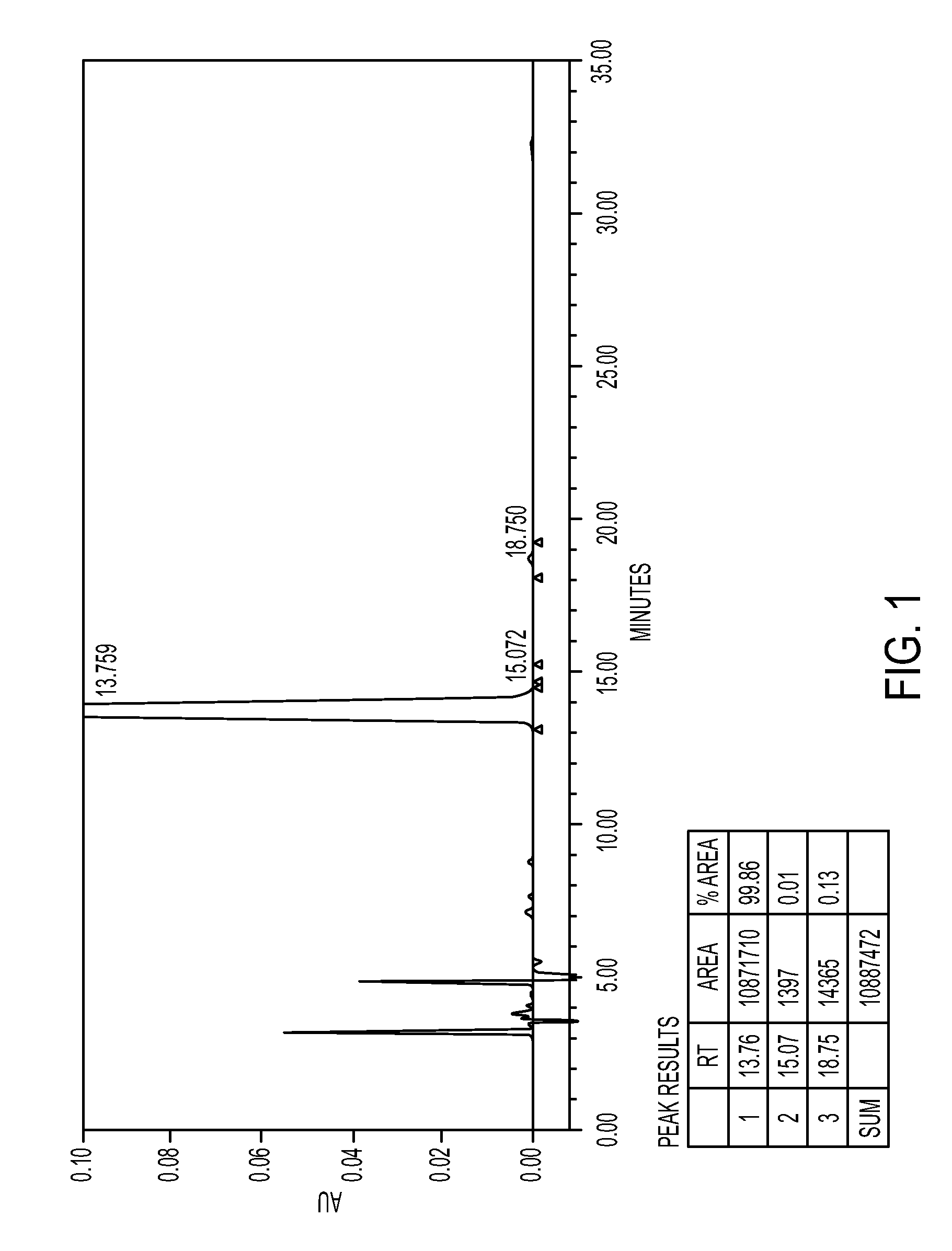
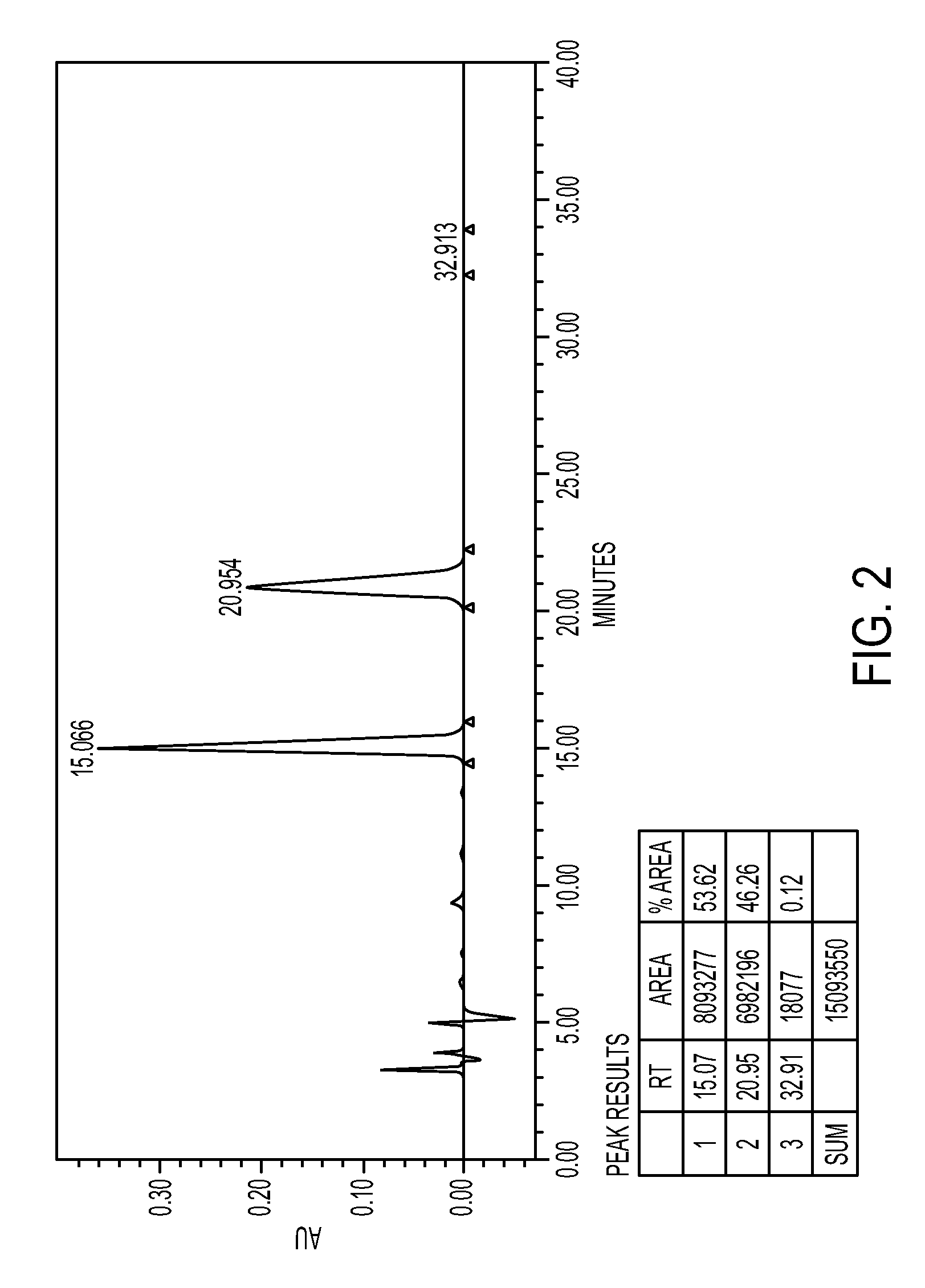
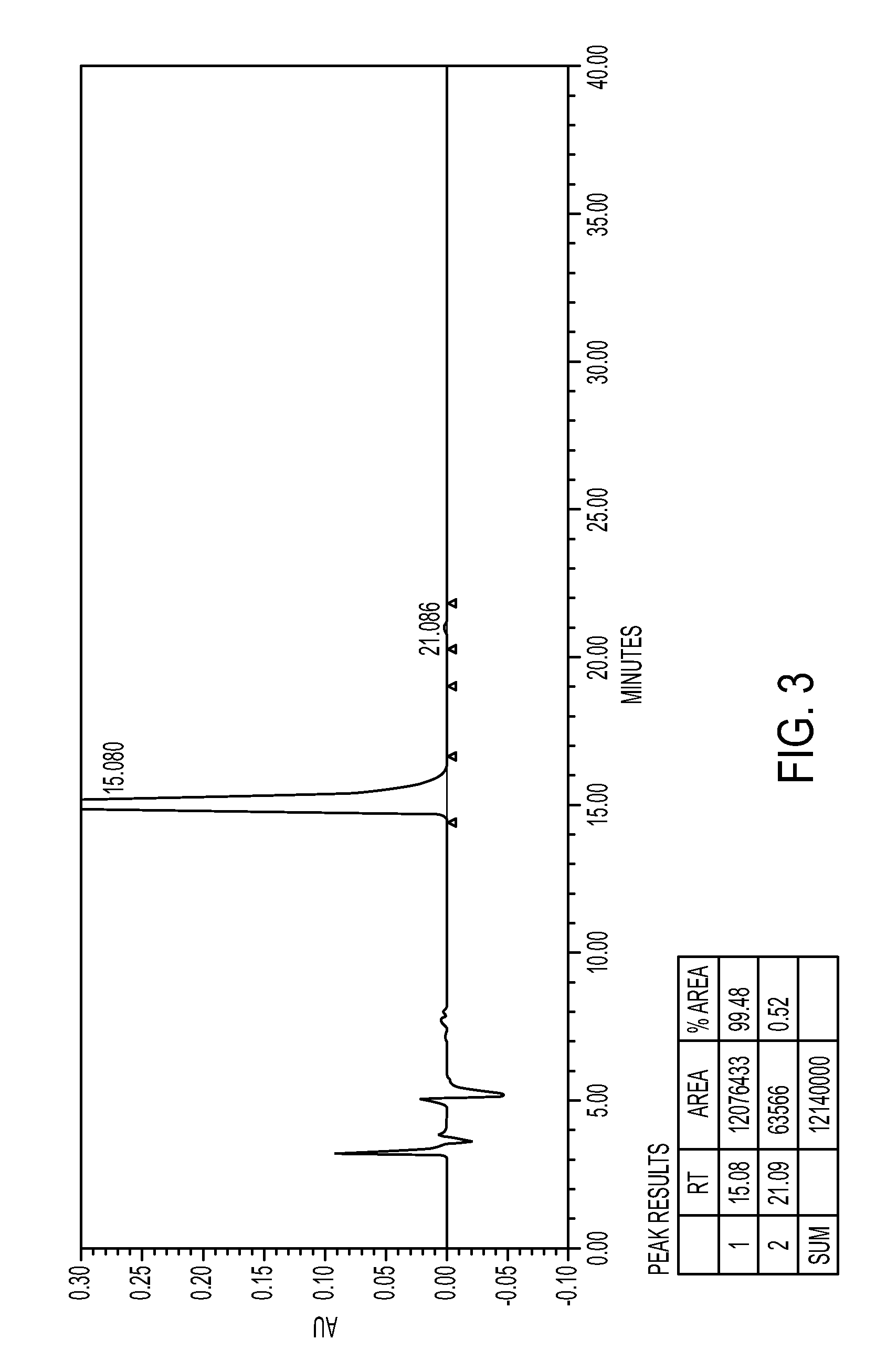
[0089]In a four necked round bottomed flask, under nitrogen atmosphere, 150 ml of ethyl acetate-methanol and 70 gm of mixture of isomers (Ratio of (7aS,2′S) / (7aR,2′S)-isomers=53.62:46.38) was taken and warmed to dissolve, and stirred for 20 hours under room temperature, crystals obtained were filtered and the solid was dried to obtain 52 gm Methyl (S)-2(2-chlorophenyl)-2-(2,4,5,6,7,7a-hexahydrothieno[3,2-c]-5-pyridin-2-one)acetate. Yield=60%; Ratio of (7aS,2′S) / (7aR,2′S)-isomers as per chiral HPLC: 99.5:0.5.
[0090]1H-NMR (DMSO-d6) spectra collected on a BRUKER 400 MHz instrument has shown values given in table 2 corresponding to structure of formula IIA free base below:
TABLE 2Chemical shiftAssignmentvalue (δ / ppm)(Multiplicity#, Number of protons, Position*)1.54-1.64(m, 1H, a)2.36-2.41(m, 1H, b)2.56-2.62(m, 1H, c)2.92-2.96(d, 1H, d)3.21-3.24(dd, 1H, e)3.66(s, 3H, f)3.86-3.89(dd, 1H, g)4....
example 3
Preparation of Methyl (7aS,2′S)-2-(2-chlorophenyl)-2-(2,4,5,6,7,7a-hexahydrothieno[3,2-c]-5-pyridin-2-one)acetate hydrogen sulfate
[0091]In a four necked round bottomed flask, under nitrogen atmosphere, 1750 ml of acetone and 70 gm of Methyl(S)-2(2-chlorophenyl)-2-(2,4,5,6,7,7a-hexahydrothieno[3,2-c]-5-pyridin-2-one)acetate isomeric mixture (Ratio of (7aS,2′S) / (7aR,2′S)-isomers=51.42:47.48) were added. It was cooled to around 5° C. and 20.8 gm of sulfuric acid was added slowly. After sulfuric acid addition, stirred at about 20-30° C. temperature. Filtered and dried under reduced pressure to obtain 84 gm of Methyl (7aS,2′S)-2(2-chlorophenyl)-2-(2,4,5,6,7,7a-hexahydrothieno[3,2-c]-5-pyridin-2-one)acetate hydrogen sulfate. Yield=93%; Purity by HPLC=99.5%, Ratio of isomers by Chiral HPLC=99.8:0.2.
[0092]1H-NMR (DMSO-d6) spectra collected on a BRUKER 400 MHz instrument has shown values given in table 3 corresponding to structure of formula IIA hydrogen sulphate below:
TABLE 3Chemical shiftA...
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com