Glucagon-like peptide-1 analogues and uses thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
I. Synthesis of Intermediates (Dimer)
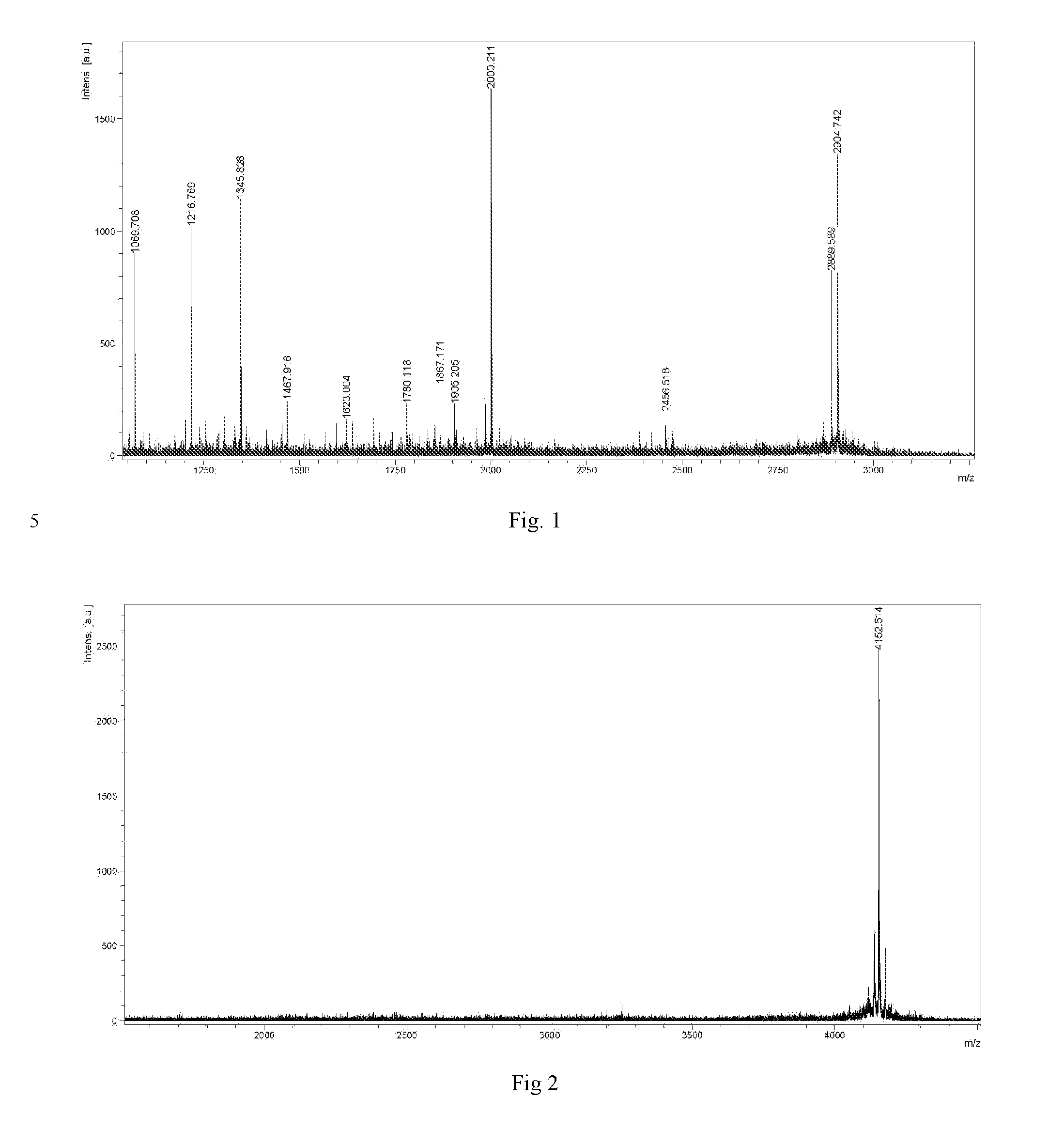
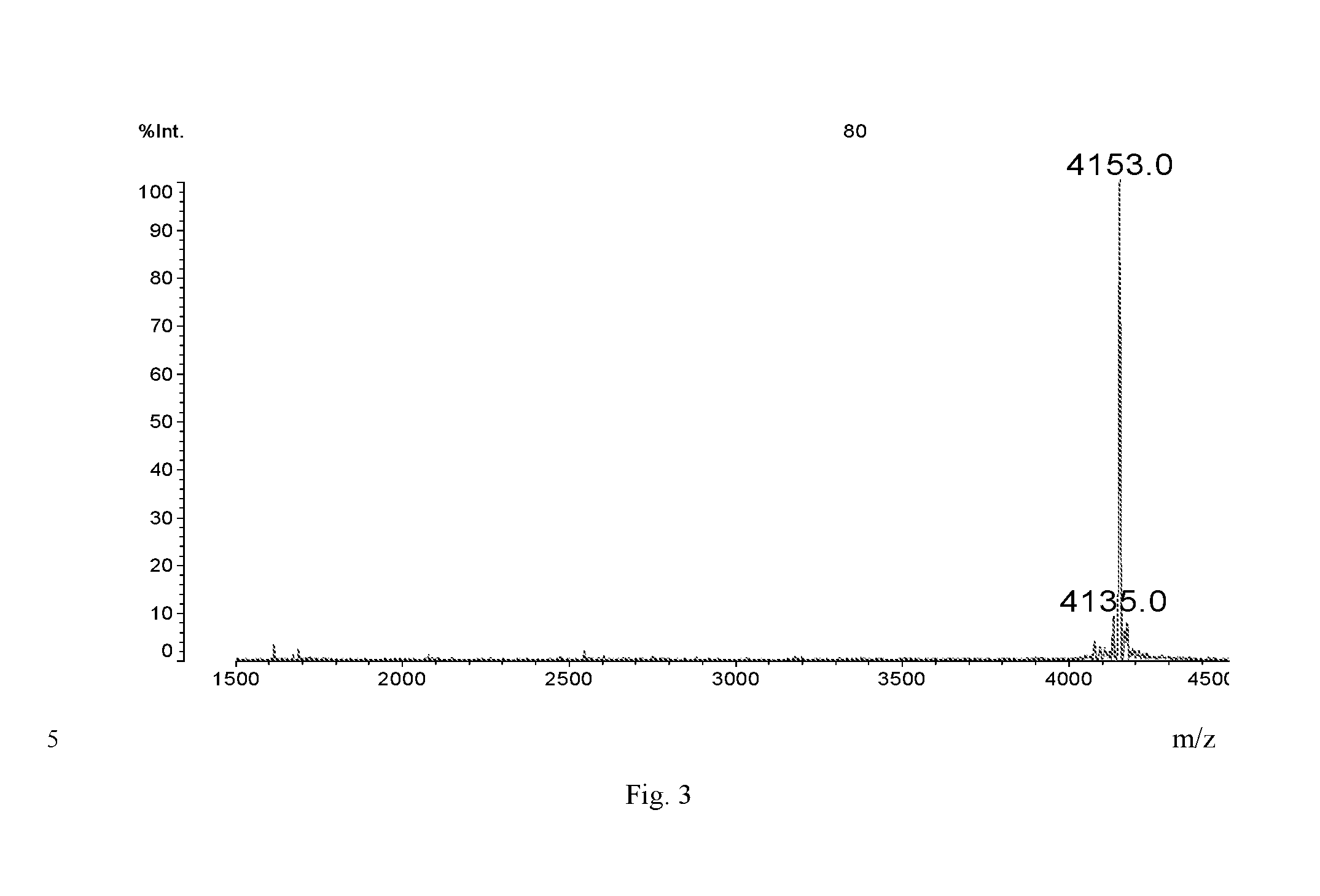
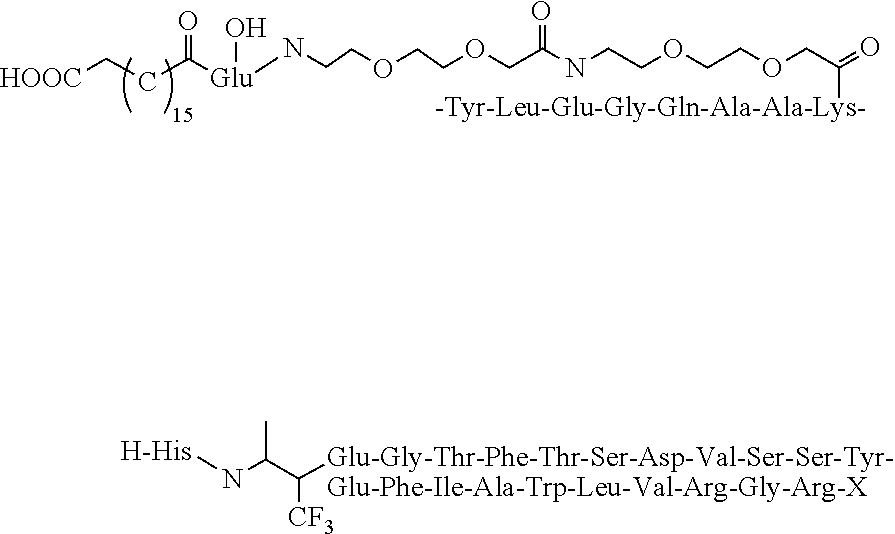
[0029]The structure of Dimer is shown below:
[0030]It can be synthesized in the following method:
1. Preparation of Compound 01.HCl
[0031]80 mL of thionyl chloride was added dropwise to 250 mL of methanol at −10° C. within 2 hours. After the mixture was warmed to the room temperature and stirred for 1 hour, 40 g of L-alanine was added and stirred overnight. The reaction mixture was heated to reflux for 4 hours, cooled to the room temperature and concentrated to give 80 g of crude compound 01.HCl.
2. Preparation of Compound 02
[0032]113 g of benzyl bromide was added to the mixture of 40 g of compound 01.HCl in 350 mL of DMF. 150 g of anhydrous potassium carbonate was added to the above mixture, and stirring was continued for 2 hours. The reaction mixture was heated to 50° C. and stirred for 2 hours, cooled to the room temperature and extracted with 400 mL of ethyl acetate and 1200 mL of water. The organic layer was washed with 150 mL of 6N HCl, the aqu...
example 2
[0069]The synthesis of the main peptide chain of Formula II was conducted by the use of Rink Amide Resin instead of Fmoc-Gly-Wang Resin. The other conditions were the same as described in Example 1.
[0070]Unless otherwise specified, the temperature referred to in Example 1 and Example 2 was the room temperature. Reagents A, B and C were 80% phenol in ethanol solution, redistilled pyridine, and a ninhydrin / ethanol solution (5 g in 100 mL).
example 3
Oral Glucose Tolerance Test (OGTT)
[0071]1. Groups
[0072]90 ICR (Institute of Cancer Research) mice, all male, were divided into three batches by body weight, with each batch having 30 mice. After fasting and overnight, each batch was divided into two groups based on the blood sugar level: the Vehicle group and the group receiving the compound of Example 1 (“Compound group”). The Vehicle group was treated with saline only, and the Compound group was treated with Compound of Example 1 dissolved in saline.
[0073]2. Experiments
[0074]Batch 1:
[0075]The mice were divided into two groups based on their blood sugar level after fasting overnight. On Day 1, they were administered with saline or the Compound in saline (0.3 mg / kg) by subcutaneous injection. 2 hours after the administration, the mice were gavaged with a dose of glucose (2 g / kg) and their blood samples were taken from the tip of the tail 30 minutes and 60 minutes after the glucose administration. Their blood glucose levels were meas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com