Engineered red-shifted channelrhodopsin variants
a technology of red-shifted channelrhodopsin and variants, which is applied in the direction of viruses, peptides, dna/rna fragmentation, etc., can solve problems such as difficulty in performing, and achieve the effects of reducing invasiveness of stimulation, improving channel kinetics, and reducing invasiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Generation of a New Channelrhodopsin Variant
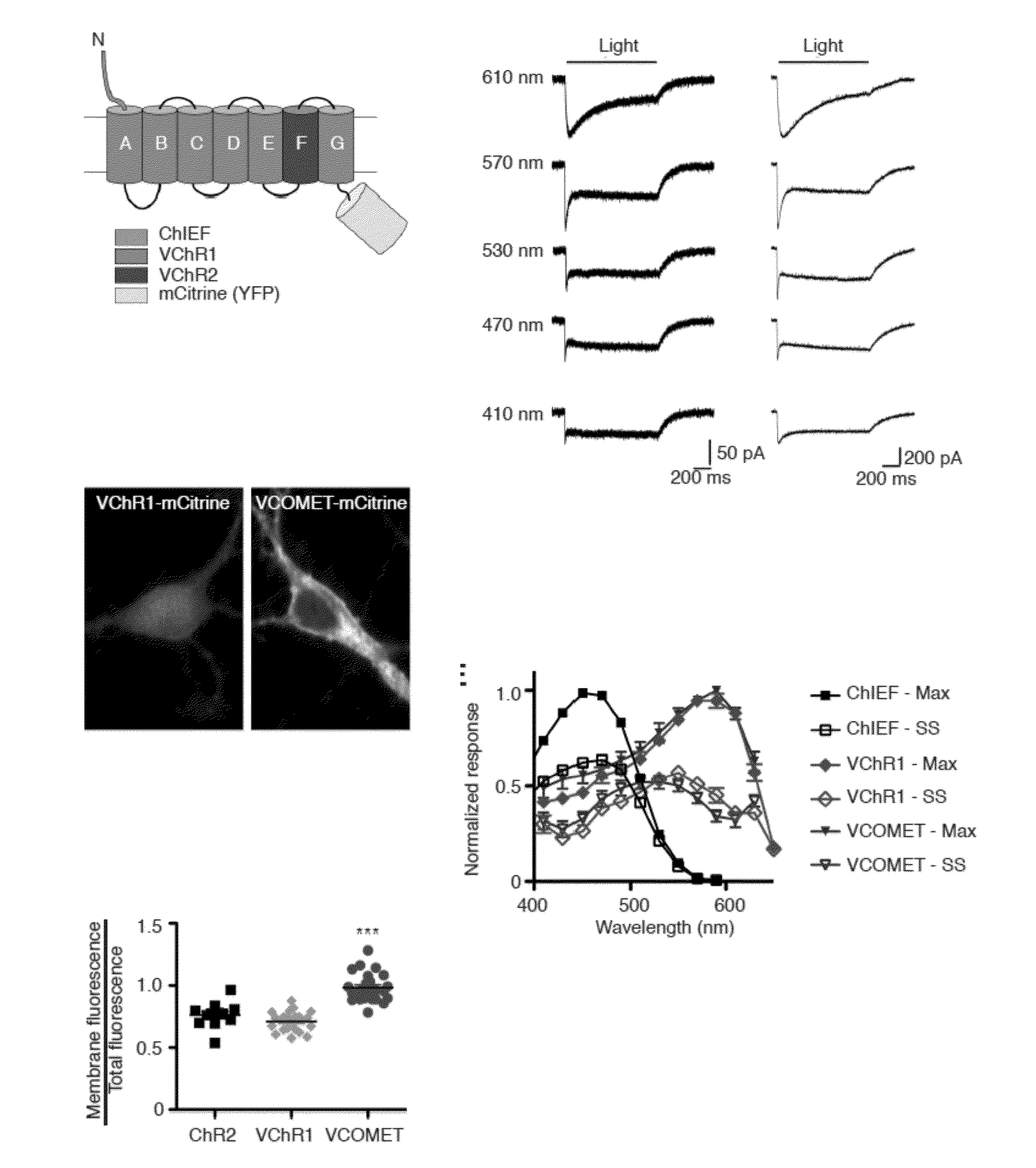
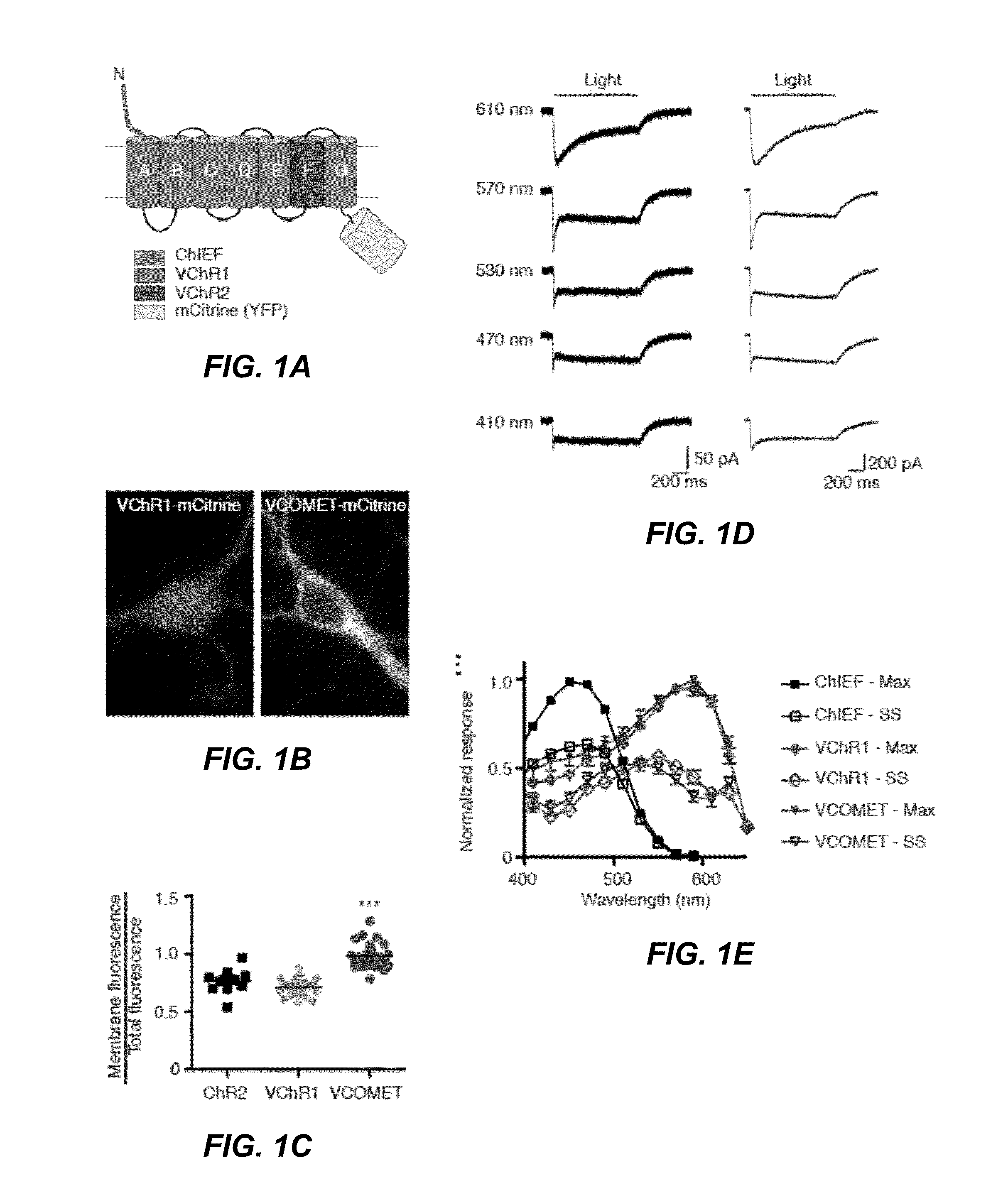
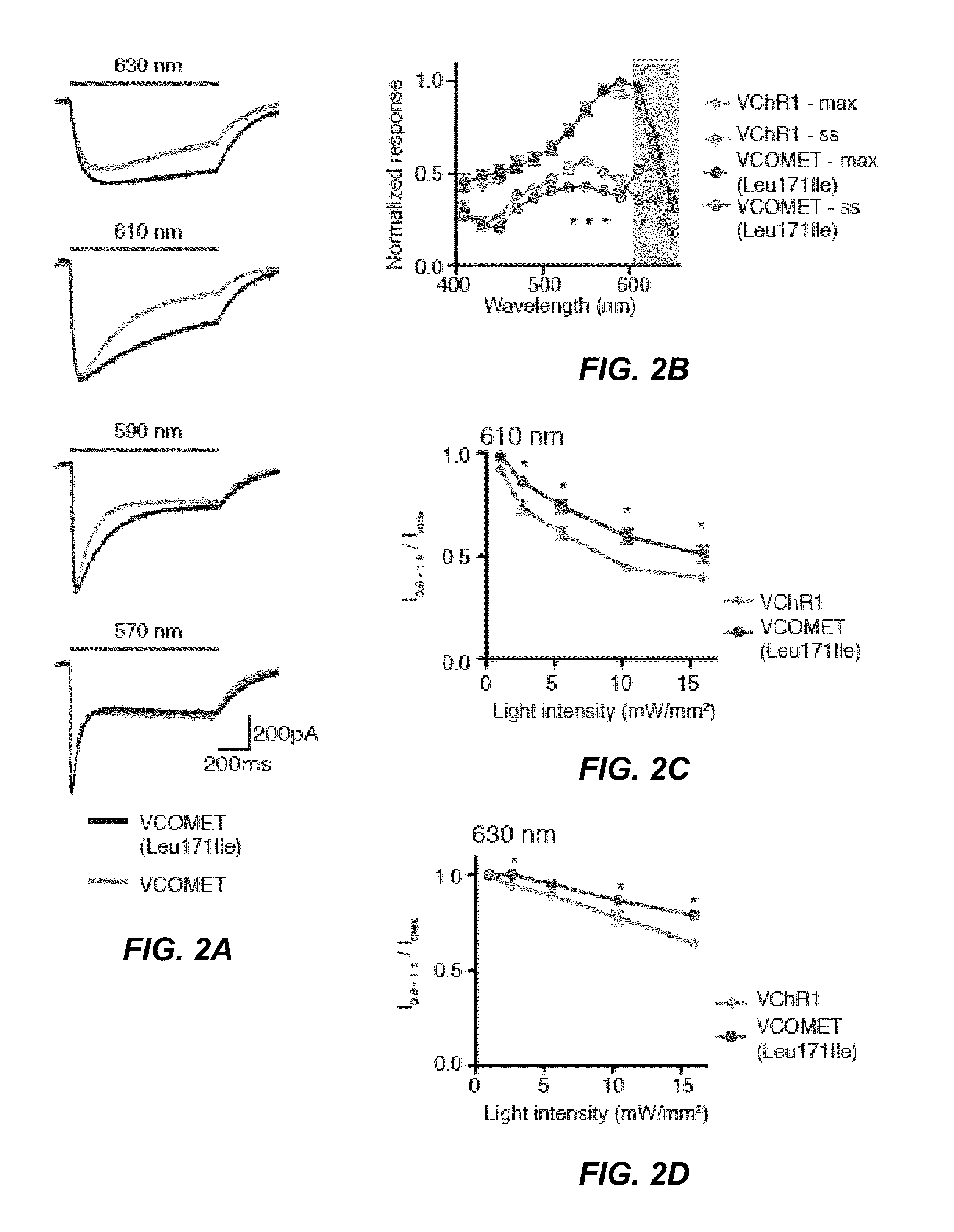
[0118]The concept of using chimeragenesis to generate new channelrhodopsin variant was conceived after the initial discovery that VChR1 poorly trafficks to the cell membrane. The chimera was conceived after comparing the membrane trafficking of ChIEF to ChR2 and VChR1. This channelrhodopsin was fused to mCitrine and tested in HEK293 cells and neurons. Improved membrane trafficking was observed as judged by localization of the fluorescence signal and increased response amplitude when conducting electrophysiology assays. The point mutations Leu171 and Glu163 were introduced to improve the kinetics of the channel, as suggested by previous studies in the channelrhodopsin ChEF and ChR2, respectively. The combination of the two mutations was found to increase the speed of termination of the light-induced response according to the electrophysiological assay. The Leu171 mutation alone was found to increase the response of VCOMET to light above 600...
example 2
Design and Engineering of a Red-Activatable Channelrhodopsin
[0119]To engineer a red-light activated channelrhodopsin, the red-shifted VChR1 channelrhodopsin variant was used as a template (Zhang, F. et al., Nat Neurosci 11, 631-633 (2008), the content of which is hereby expressly incorporated by reference in its entirety for all purposes). VChR1 expresses poorly in mammalian cells and has minimal trafficking to the membrane (Yizhar, O. et al., Nature 477, 171-178 (2011); and Lin, J. Y., Exp Physiol 96, 19-25 (2011)) resulting in small photocurrents, e.g., <50 pA, that cannot be accurately characterized.
[0120]To improve membrane trafficking of the engineered channelrhodopsin, the N-terminal sequence prior to the first transmembrane domain of VChR1 was replaced with the corresponding ChIEF sequence, denoted C-VChR1 (FIG. 6A), which improves its membrane trafficking (Lin, J. Y., Exp Physiol 96, 19-25 (2011)) (FIGS. 6B and C). This strategy is based on the superior membrane trafficking ...
example 3
Lentivirus and Recombinant Adeno-Associated Virus (rAAV) Production
[0127]ReaCh-mCitrine was subcloned into a generation 2 lentiviral construct with an hSynapsin promoter. The ReaCh-mCitrine lentivirus was made according to the protocols published on the Salk Institute's Gene Transfer Targeting and Therapeutics Core website, with minor modification. Briefly, 293A cells (Life Technologies, Carlsbad, Calif.) were grown to 85% confluence and transfer vector containing ReaCh-mCitrine, psPAX2, and pMD2.G (gifts from Dr. Didier Trono, Ecole Polytechnique Fédérale de Lausanne) were transfected with calcium phosphate approach (Clontech, Mountain View, Calif.). Virus particles were harvested from serum-free medium and concentrated with 20% sucrose cushion with ultracentrifugation. The titer of lentivirus was estimated with Lentivirus Rapid Quantitation Kit (Cell Biolabs Inc. San Diego Calif., USA) to be 1.7×109 virus particles / mL. The Lentiviral vector was a gift from Dr. Ed Boyden, MIT).
[012...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com