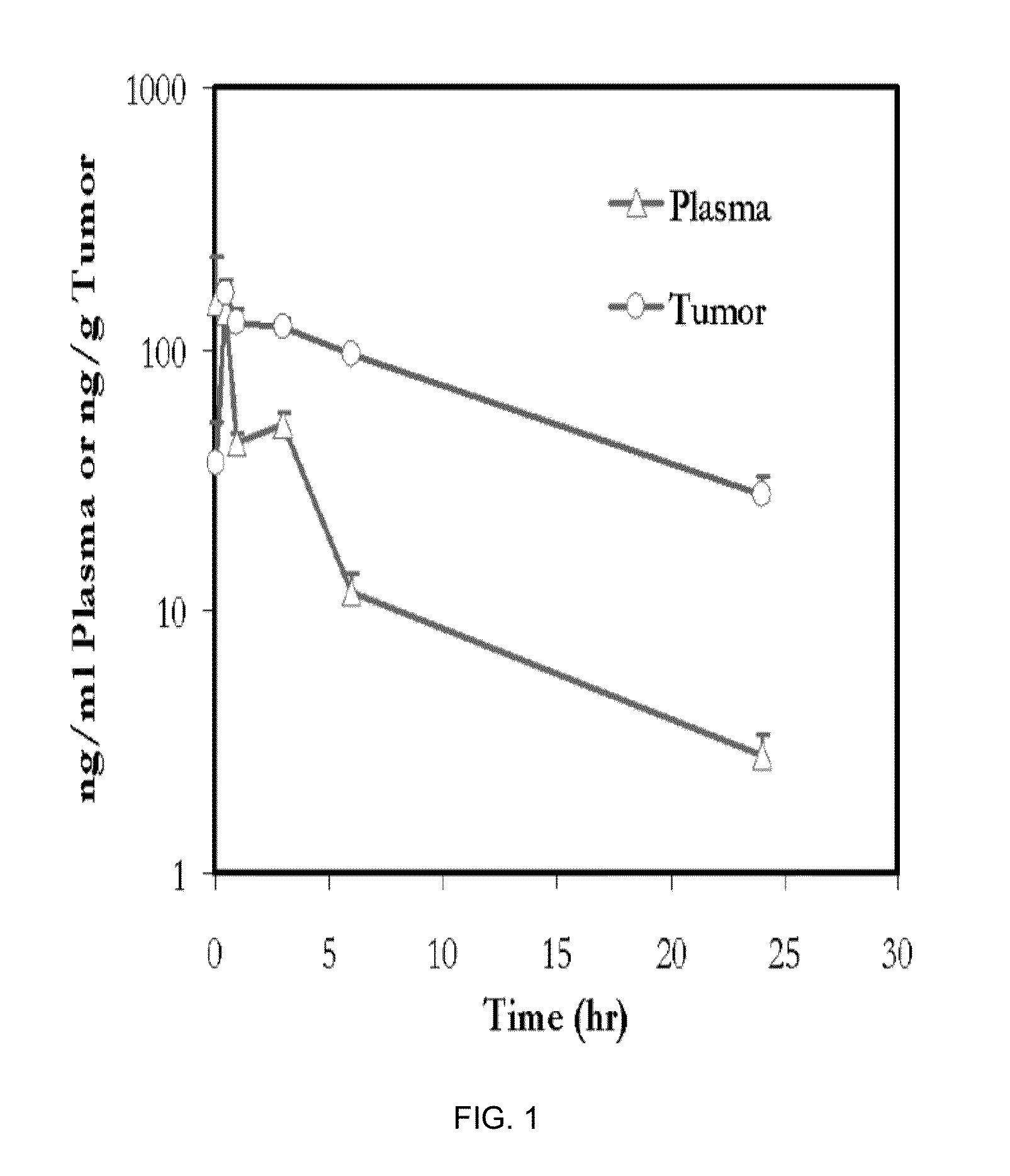
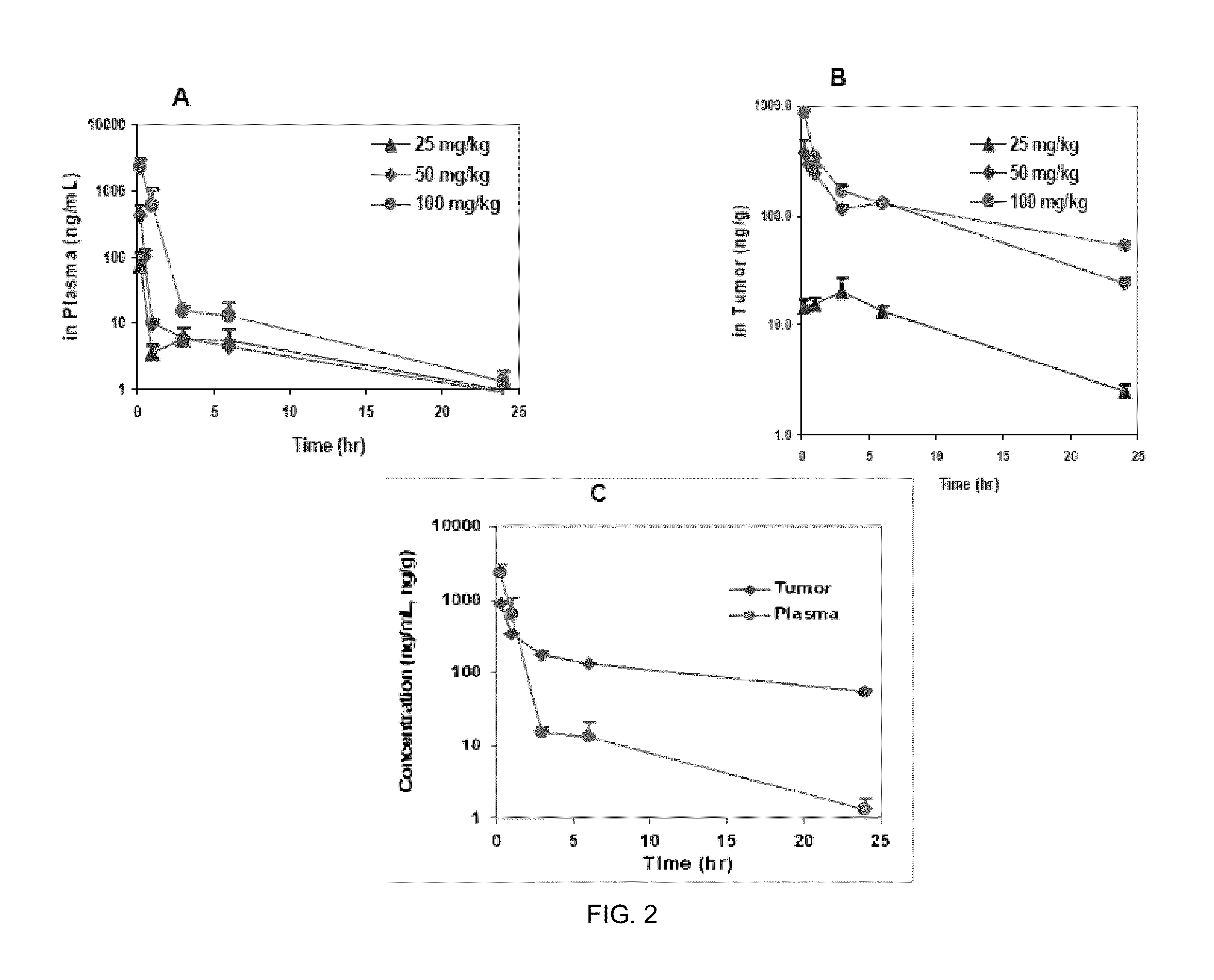
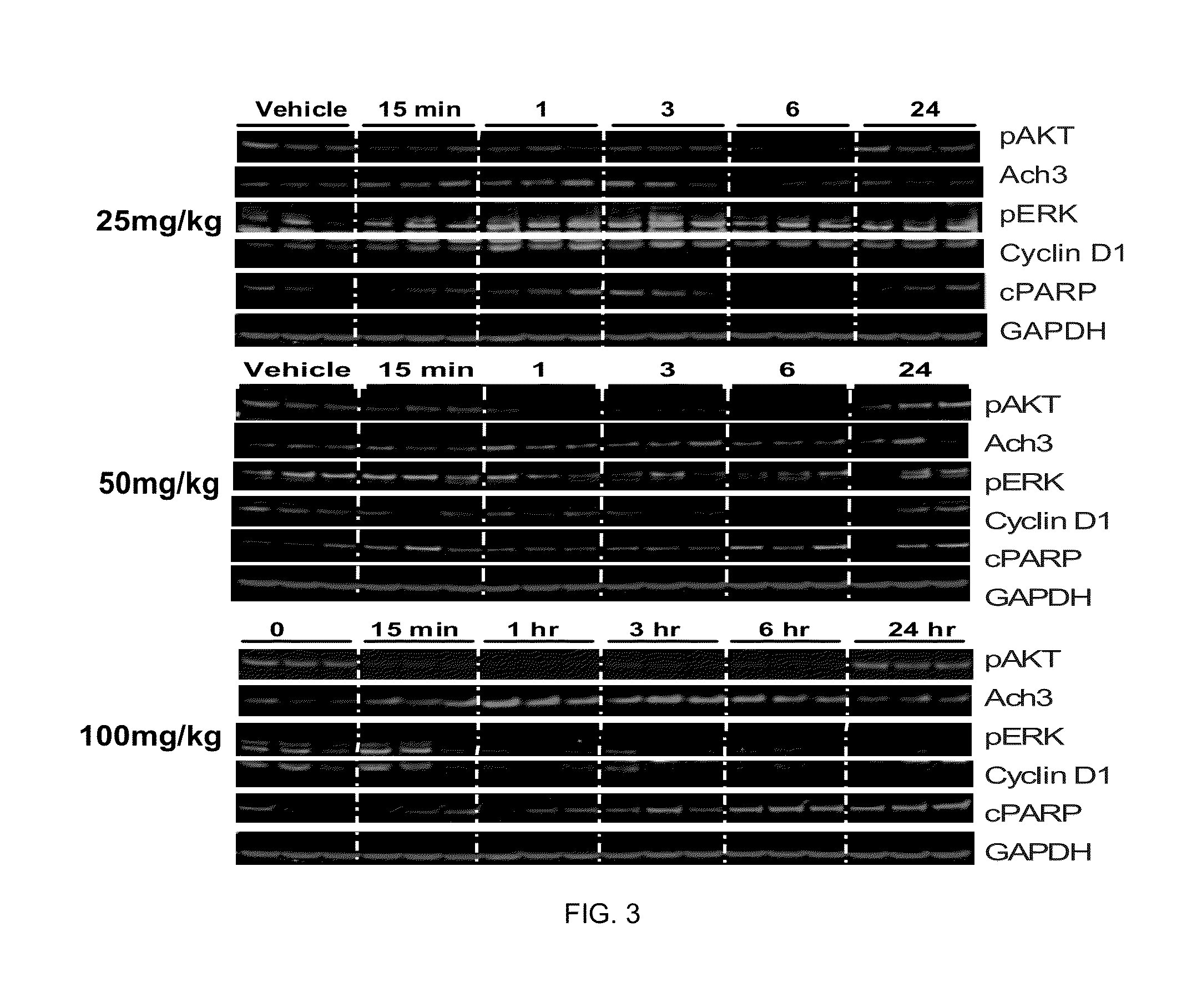
Phosphoinositide 3-kinase inhibitor with a zinc binding moiety
a phosphoinositide and zinc binding technology, applied in the field of phosphoinositide 3kinase inhibitors with zinc binding moiety, can solve the problems of low in vivo anti-tumor activity, oncogenic transformation and cancer, and treatment regimes using a cocktail of cytotoxic drugs often are limited by dose-limiting toxicities, so as to achieve potent antiproliferative activity, potent inhibitory activity, and advantageous properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of N-hydroxy-2-(((2-(6-methoxypyridin-3-yl)-4-morpholinothieno[3,2-d]pyrimidin-6-yl)methyl)(methyl)amino)pyrimidine-5-carboxamide (Compound 1)
Step a: (Z)-Ethyl-2-(ethoxymethyl)-3-methoxyacrylate (Compound 202)
[0138]Sodium (40.9 g, 1.78 mol) was added to ethanol (750 mL) in portions carefully and the solution was concentrated to give NaOEt powder after all sodium metal disappeared. Under stirring, hexane (1.0 L) was added and the mixture was cooled with ice-water bath. A mixture of 201 (130 g, 0.89 mol) and ethyl formate (131 g, 1.78 mol) was added dropwise at 0-5° C. The reaction mixture was stirred at room temperature overnight. Dimethyl sulfate (224 g, 1.78 mol) was added dropwise with cooling of ice-water bath. The resulting mixture was heated at 50° C. for 2 h. To the mixture was added triethylammonium chloride (122 g) and sodium hydroxide (20 g). The mixture was then stirred at room temperature for 4 h and filtered. The filtrate was washed with water and dried over ...
example 2
Preparation of N-hydroxy-2-(((2-(6-methoxypyridin-3-yl)-4-morpholinothieno[3,2-d]pyrimidin-6-yl)methyl)(methyl)amino)pyrimidine-5-carboxamide methanesulfonate (Compound 2)
[0167]Method A: To a mixture of Compound 1 (300 mg, 0.59 mmol) and MeOH / Et2O (3 / 1, 40 mL) was added a solution of methanesulfonic acid (114 mg, 1.18 mmol) in MeOH (3 mL) at 0° C. The resulting mixture was stirred at 0° C. for 3 h. The precipitate was collected by filtration and washed with Et2O to afford Compound 2 as a white solid (260 mg, 73%).
[0168]Method B: To a suspension of Compound 1 (1.5 g, 2.95 mmol) in dichloromethane / MeOH (40 mL / 10 mL) was added methanesulfonic acid (341 mg, 3.55 mmol) in 2 mL MeOH at room temperature (15° C.) to form a clear solution. The reaction mixture was stirred at room temperature overnight. The reaction mixture was still clear. Ethyl acetate (40 mL) was added to the mixture and continued to stir for 3 h at room temperature. The resulting precipitate was collected by filtration to...
example 3
Preparation of N-hydroxy-2-(((2-(6-methoxypyridin-3-yl)-4-morpholinothieno[3,2-d]pyrimidin-6-yl)methyl)(methyl)amino)pyrimidine-5-carboxamide sodium salt (Compound 3)
[0170]To a suspension of Compound 1 (300 mg, 0.59 mmol) in methanol (30 mL) at 0° C. was added slowly t-BuONa (85 mg, 0.88 mmol). The resulting mixture was warmed to room temperature and continued to stir for 2 h. The reaction was concentrated and the residue was triturated and washed with ethanol followed by filtration to afford Compound 3 as a white solid (230 mg, 73%). m.p.: 178-183° C. LCMS: 509.3 [M+1]+. 1H NMR (400 MHz, DMSO-d6): δ 3.17 (s, 3H), 3.75 (s, 4H), 3.92 (s, 7H), 5.16 (s, 2H), 6.90 (d, J=8.4 Hz, 1H), 7.42 (s, 1H), 8.57 (d, J=8.0 Hz, 1H), 8.65 (s, 2H), 9.14 (s, 1H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| body weight | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com