Method of analyzing hemoglobins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
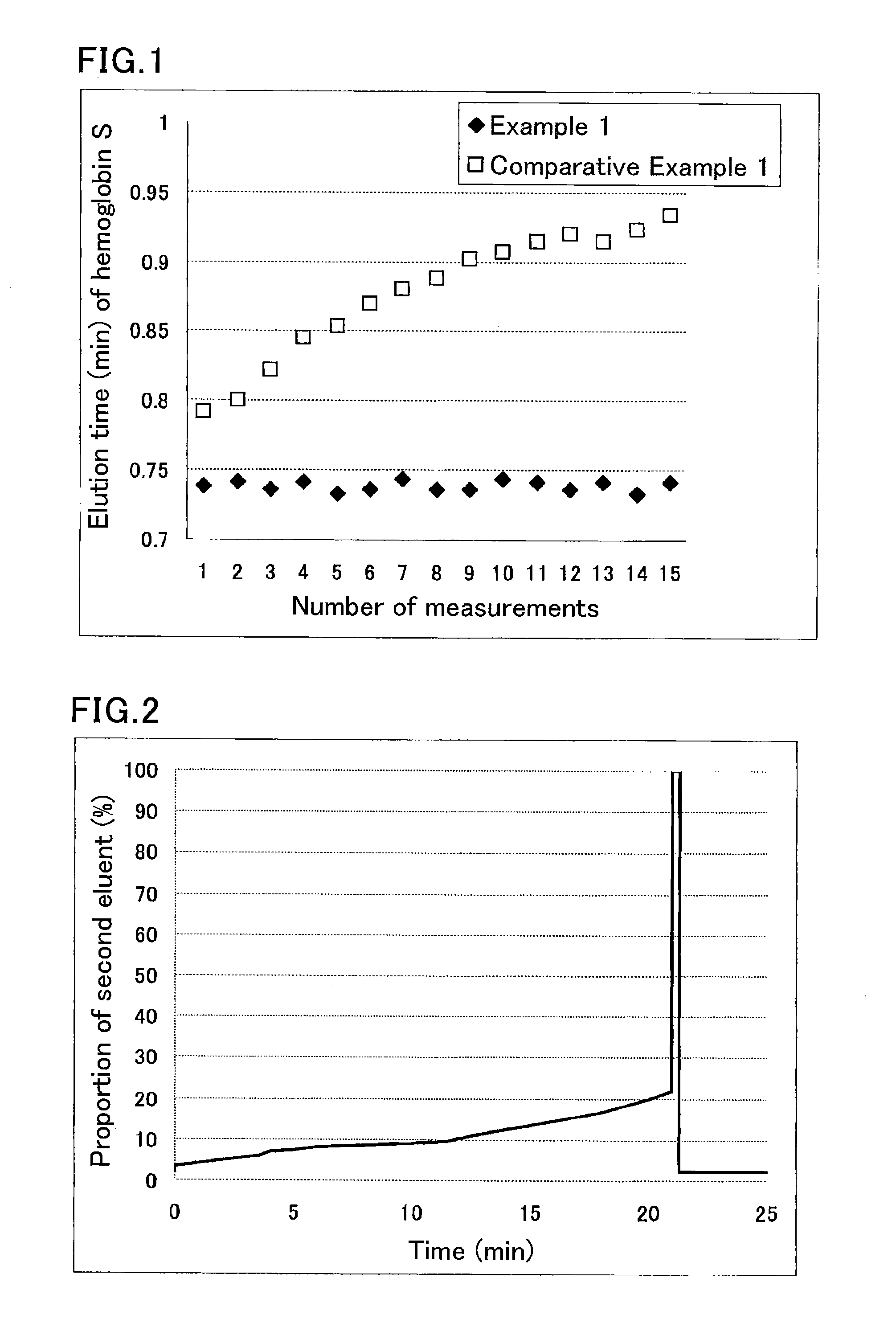
[0075]A sample was prepared by diluting a hemoglobin S-containing blood sample 100-fold with a sample pre-treatment solution (phosphate buffer (pH 7.0) containing 0.1% by weight Triton X-100).
[0076]The used separation column was a column containing cation-exchange resin filler particles having sulfonic acid groups on the surfaces.
[0077]The used HPLC instrument was provided with an autosampler SIL-20AC (Shimadzu Corp.), a delivery pump LC-20AD (Shimadzu Corp.), a degasser DGU-20A5 (Shimadzu Corp.), a column oven CTO-20AC (Shimadzu Corp.), and a detector SPD-M20A (Shimadzu Corp.). The instrument was run under the following conditions:
[0078]eluent flow rate: 1.7 mL / min;
[0079]detection wavelength: 415 nm; and
[0080]amount of introduced sample: 10 μL.
[0081]Each portion of the sample was eluted and measured using the following eluents for the respective periods of time:
[0082]until 0.5 minutes after the start: eluent 1 (40 mmol / L phosphate buffer (pH 5.4) containing 60 mmol / L sodium perchlo...
example 2
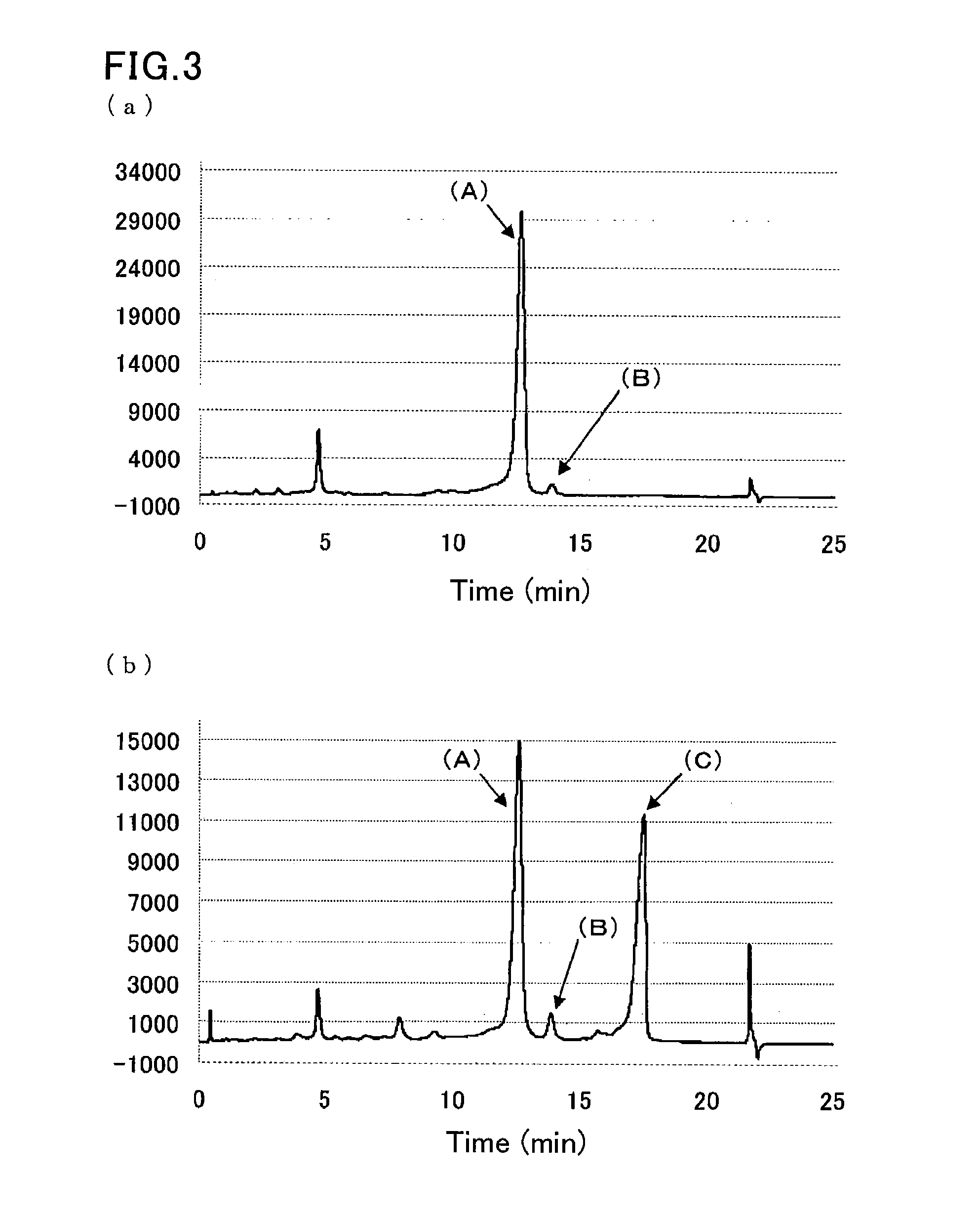
[0090]A sample was prepared by dissolving glycohemoglobin control level II (Sysmex Corp.) in water for injection (200 μL), and further diluting the solution 100-fold with a sample pre-treatment solution (10 mmol / L phosphate buffer (pH 7.0) containing 0.1% by weight Triton X-100).
[0091]Another sample was prepared by diluting a hemoglobin S-containing blood sample 100-fold with the sample pre-treatment solution (10 mmol / L phosphate buffer (pH 7.0) containing 0.1% by weight Triton X-100).
[0092]The same separation column as that of Example 1 was used.
[0093]The same HPLC instrument as that of Example 1 was run under the following conditions:
[0094]flow rate: 1.7 mL / min;
[0095]detection wavelength: 415 nm; and
[0096]amount of introduced sample: 10 μL.
[0097]Each sample was eluted and measured by linear gradient of two eluents:
[0098]first eluent: eluent 6 (20 mmol / L phosphate buffer (pH 5.4) containing 30 mmol / L sodium perchlorate, 1 mmol / L sodium nitrite, and 1 mmol / L sodium azide); and
[0099]...
example 3
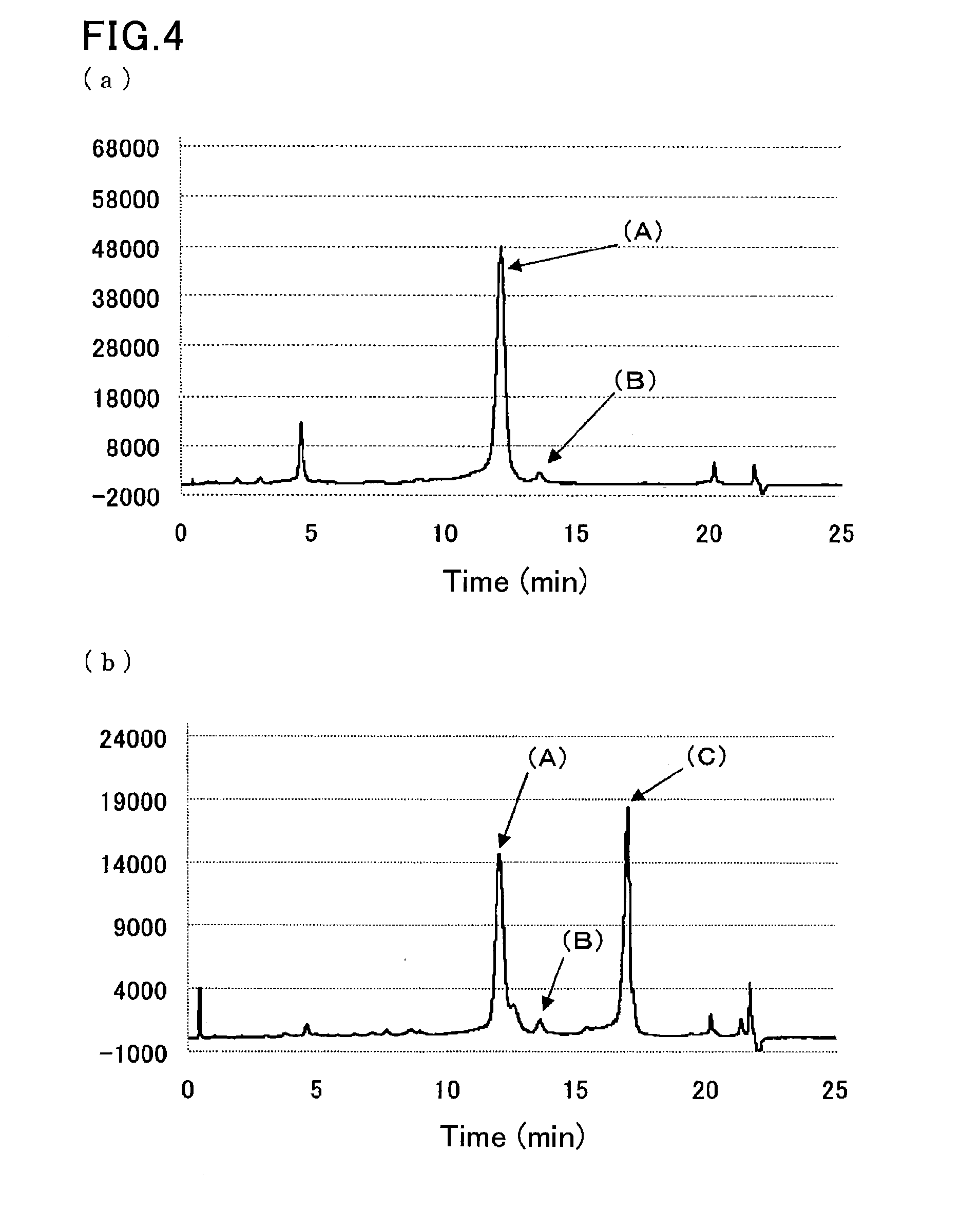
[0101]A sample was prepared by dissolving glycohemoglobin control level II (Sysmex Corp.) in water for injection (200 μL), and further diluting the solution 100-fold with a sample pre-treatment solution (10 mmol / L phosphate buffer (pH 7.0) containing 0.1% by weight Triton X-100, 1 mmol / L sodium nitrite, and 1 mmol / L sodium azide).
[0102]Another sample was prepared by diluting a hemoglobin S-containing blood sample 100-fold with the sample pre-treatment solution (10 mmol / L phosphate buffer (pH 7.0) containing 0.1% by weight Triton X-100, 1 mmol / L sodium nitrite, and 1 mmol / L sodium azide).
[0103]The samples were measured in the same manner as in Example 2, except that eluent 3 (40 mmol / L phosphate buffer (pH 8.0) containing 0.8% by weight Triton X-100, 300 mmol / L sodium perchlorate, and 1 mmol / L sodium azide) used in Example 1 was used as the second eluent. FIG. 4 are the resulting chromatograms.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com