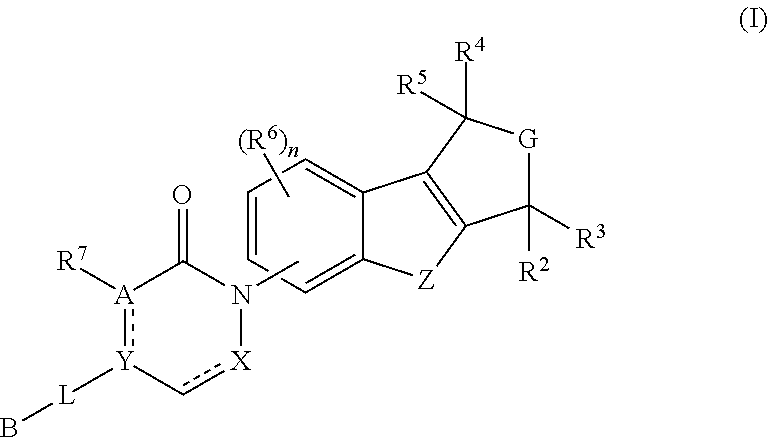
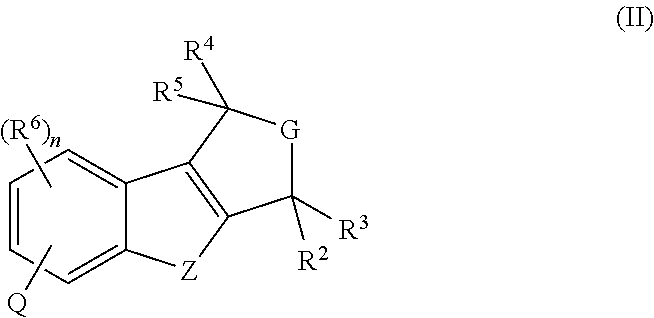
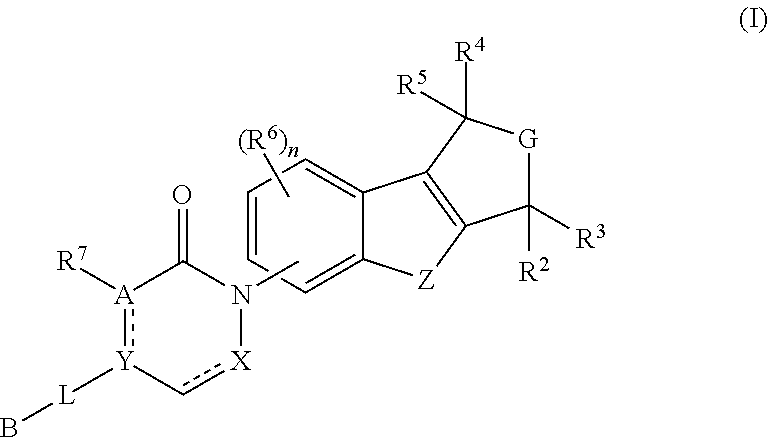
Pyrido-/azepino-benzofuran and pyrido-/azepino-benzothiophene mch-1 antagonists, methods of making, and use thereof
a technology of pyridobenzothiophene and azepinobenzothiophene, which is applied in the field of pyrido/azepinobenzofuran and pyrido/azepinobenzothiophene mch1 antagonists, methods of making, can solve the problems of limited efficacy, current available pharmaceutical therapies for the treatment of obesity, and limit their use, so as to increase body mass index, increase waist circum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Analytical Methods and Materials
[0150]Unless otherwise noted, reagents and solvents were used as received from commercial suppliers. Proton nuclear magnetic resonance (NMR) spectra were obtained on Bruker spectrometers at 300, 400 or 500 MHz. Spectra are given in ppm (δ) and coupling constants, J, are reported in Hertz. Tetramethylsilane (TMS) was used as an internal standard. Mass spectra were collected using either a Finnigan LCQ Duo LCMS ion trap electrospray ionization (ESI) or a mass Varian 1200L single quadrapole mass spectrometer (ESI). High performance liquid chromatograph (HPLC) analyses were obtained using a Luna C18(2) column (250×4.6 mm, Phenomenex) or a Zorbax Bonus-RP column (150×4.6 mm, 3.5 um, Agilent) with UV detection at 254 nm or 223 nm using a standard solvent gradient program (Method A, Method B or Method C).
Method A:
[0151]
TimeFlow(min)(mL / min)% A% B0.01.0982201.0298251.0298271.0982A = Water with 0.025% Trifluoroacetic AcidB = Acetonitrile with 0.025% Trifluoroa...
example 2
Preparation of 4-(Benzyloxy)-1-(1,2,3,4-tetrahydrobenzofuro[2,3-c]pyridin-7-yl)pyridin-2(1H)-one hydrochloride
a) O-(3-Bromophenyl)hydroxylamine hydrochloride
[0154]CAS Registry Number 937716-47-7
[0155]Potassium hydroxide (7.70 g, 137 mmol) was added to a biphasic solution of 3-bromophenol (19.0 g, 110 mmol) in toluene (27.0 mL), iPrOH (16.4 mL) and H2O (2.67 mL). The resulting suspension was heated at reflux for 1.5 h. A solution of hydroxylamine-O-sulfonic acid (3.10 g, 27.5 mmol) in H2O (16.4 mL) was added dropwise over 20 min to the refluxing solution. The solution was allowed to stir for an additional 15 min. The solution was placed in an ice bath for 5 min. The solution was diluted with 10% NaOH (aq) (100 mL) and with CH2Cl2 (200 mL). The resulting layers were separated, and the aqueous phase was extracted with CH2Cl2 (2×75 mL). The combined organic extracts were washed with 10% NaOH (aq) (7×100 mL), dried over Na2SO4, filtered and partially concentrated under reduced pressure. ...
example 3
Preparation of 4-((5-Fluoropyridin-2-yl)methoxy)-1-(1,2,3,4-tetrahydrobenzofuro[2,3-c]pyridin-7-yl)pyridin-2(1H)-one hydrochloride
a) 4-((5-Fluoropyridin-2-yl)methoxy)pyridin-2(1H)-one
[0164]CAS Registry Number 924311-90-0
[0165]This compound was prepared in accordance with the procedure described in PCT Publication No. WO 2009 / 089482 to Guzzo et al., which is hereby incorporated by reference in its entirety.
b) tert-Butyl 7-(4-((5-fluoropyridin-2-yl)methoxy)-2-oxopyridin-1(2H)-yl)-3,4-dihydrobenzofuro[2,3-c]pyridine-2(1H)-carboxylate
[0166]
[0167]tert-Butyl 7-bromo-3,4-dihydrobenzofuro[2,3-c]pyridine-2(1H)-carboxylate (0.15 g, 0.43 mmol), 4-((5-fluoropyridin-2-yl)methoxy)pyridin-2(1H)-one (0.11 g, 0.51 mmol), and Cs2CO3 (0.18 g, 0.55 mmol) were suspended in toluene (6 mL), and N2 was bubbled through the suspension for 15 min. The suspension was treated with (1S,2S)N,N′-bismethyl-1,2-cyclohexane-diamine (0.10 mL, 0.63 mmol) and bubbled with N2 for 5 min. Copper iodide (0.12 g, 0.63 mmol) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com