Aqueous coloring agent dispersion for inkjet, ink composition, inkjet recording method, and colored body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
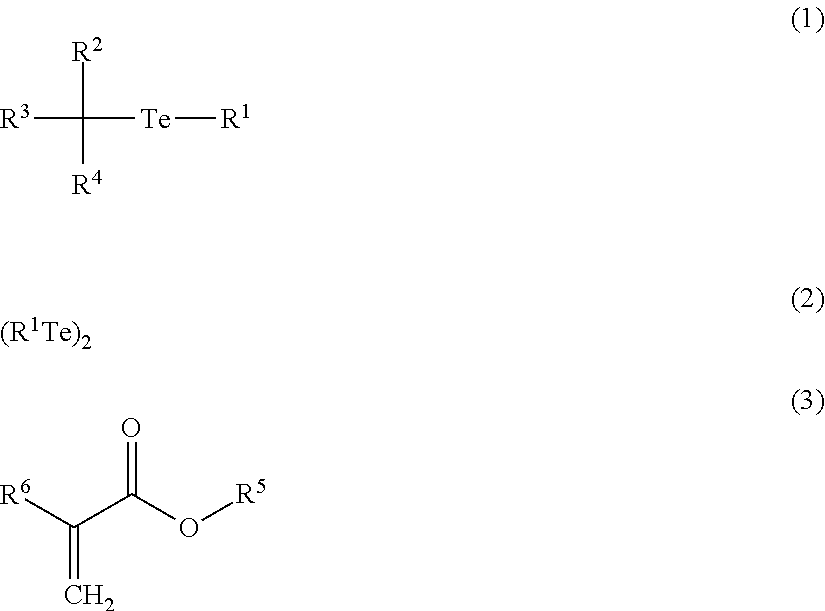
Synthesis of Ethyl-2-Methyl-2-n-Butyltellanyl-Propionate (Hereinafter, Referred to as “BTEE”)
[0167]6.38 g (50 mmol) of metal tellurium (trade name: Tellurium (−40 mesh), manufactured by Aldrich Corporation) was suspended in 50 ml of THF. 34.4 mL (55 mmol) of n-butyllithium (manufactured by Aldrich Corporation, 1.6 M hexane solution) were gradually added dropwise to the obtained suspension at room temperature (for 10 minutes). The obtained reaction solution was stirred until the metal tellurium was completely dissolved (for 20 minutes). Next, 10.7 g (55 mmol) of ethyl-2-bromo-isobutyrate were added at room temperature and stirred for 2 hours. After completion of the reaction, the solvent was concentrated under reduced pressure, and subsequently, was distilled under reduced pressure, thereby obtaining 8.98 g (yield: 59.5%) of a yellow oily substance of BTEE.
synthesis example 2
Synthesis of Dibutyl Ditelluride (Hereinafter, Referred to as “DBDT”)
[0168]3.19 g (25 mmol) of metal tellurium (trade name: Tellurium (−40 mesh), manufactured by Aldrich Corporation) was suspended in 25 ml of THF. 17.2 mL (27.5 mmol) of n-butyllithium (manufactured by Aldrich Corporation, 1.6 M hexane solution) were gradually added dropwise to the obtained suspension at 0° C. (for 10 minutes). The obtained reaction solution was stirred until the metal tellurium was completely dissolved (for 10 minutes). Next, 20 mL of ammonium chloride solution were added at room temperature and stirred for 1 hour. After completion of the reaction, the organic layer was separated and the water layer was extracted 3 times with diethyl ether. The collected organic layer was dried with anhydrous sodium sulfate and then concentrated under reduced pressure, thereby obtaining 4.41 g (11.93 mmol; yield: 95%) of a grape oily substance of DBDT.
(B) Preparation of Block Copolymer
synthesis example 3
Synthesis of Block Copolymer A
[0169]In a nitrogen-replaced glove box, 90 g (511 mmol) of benzyl methacrylate (manufactured by Tokyo Chemical Industry Co., Ltd.), 2.00 g (6.67 mmol) of BTEE, 1.22 g (3.33 mmol) of DBDT, 0.33 g (2.00 mmol) of 2,2′-azobis-isobutyronitrile (trade name: AIBN, manufactured by Otsuka Chemical Co., Ltd., hereinafter referred to as “AIBN”), and 90 g of methoxypropanol were charged into a flask equipped with a stirrer, and then reacted at 60° C. for 16 hours. The polymerization rate was 99.6%, the Mw was 16,200, and the PDI was 1.41.
[0170]45 g (317 mmol) of butyl methacrylate (manufactured by Tokyo Chemical Industry Co., Ltd.), 25 g (290 mmol) of methacrylic acid (manufactured by Tokyo Chemical Industry Co., Ltd.), 0.22 g (1.33 mmol) of AIBN, and 70 g of methoxypropanol were added to the obtained solution, and then reacted at 60° C. for 22 hours. The polymerization rate was 99.1%.
[0171]After completion of the reaction, the reaction solution was poured into 5 L...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Dispersion potential | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com