Production method for hematite for iron production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
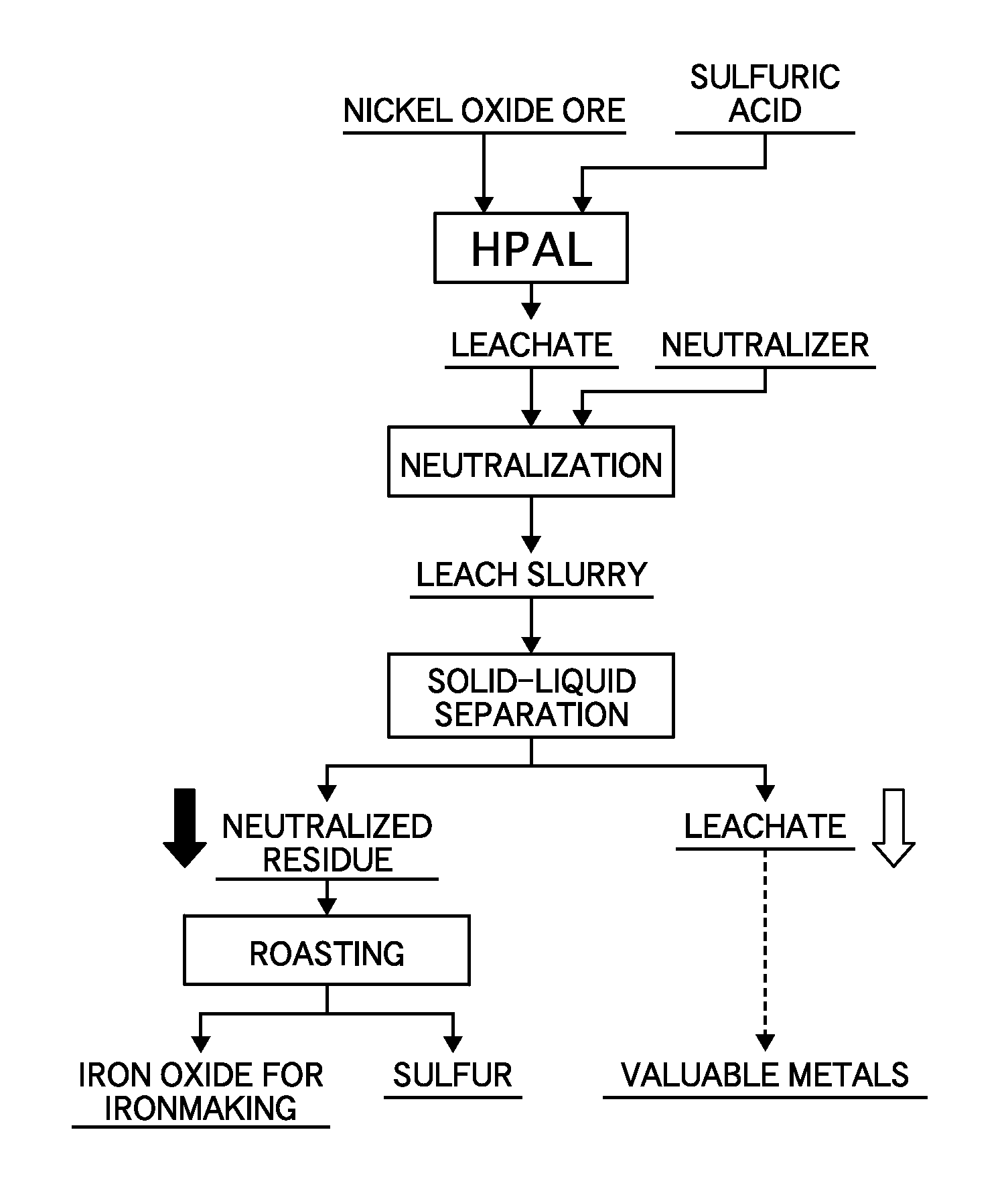
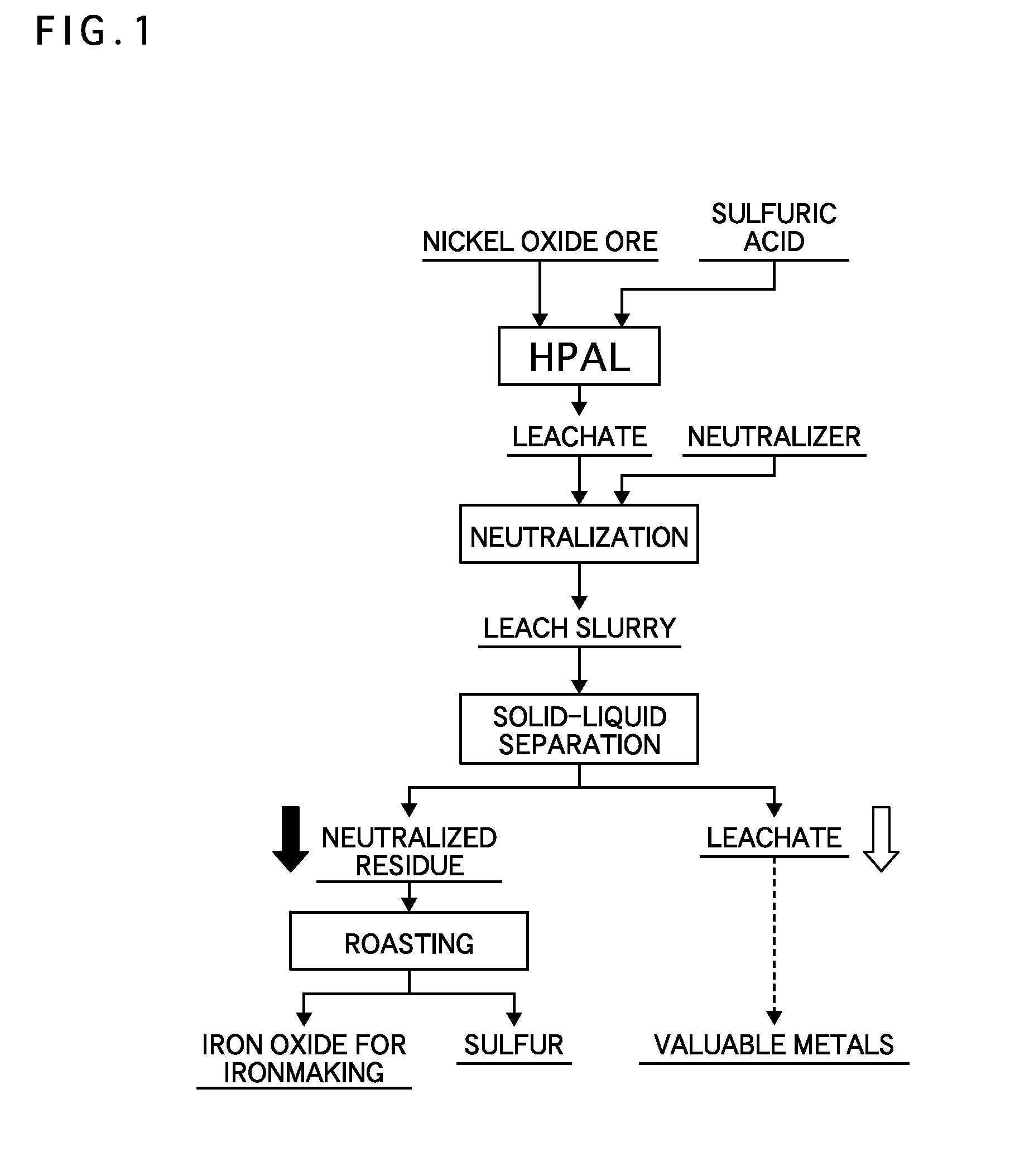
[0042]Nickel oxide ore having 1% nickel grade and 46 to 48% iron grade was adjusted to be a slurry of 30 to 40% by weight, and then was mixed with sulfuric acid of 64% by weight. Subsequently, the slurry was charged into a pressure device, heated to 240 to 250° C., and maintained for one hour, and a leachate was obtained by leaching nickel in the ore (HPAL).
[0043]After the leaching, the leachate was cooled to about 70° C., and then slaked lime was added to neutralize a surplus acid (neutralization). The slurry containing a leach residue after the surplus acid was neutralized (hereinafter the leach residue after the neutralization is referred to as “neutralized residue’) was subjected to solid-liquid separation using Nutsche and a filtering bottle, and was separated into the leachate and the neutralized residue (solid-liquid separation).
[0044]In the neutralized residue, an iron grade was 49.9%, and a sulfur grade was 1.5%.
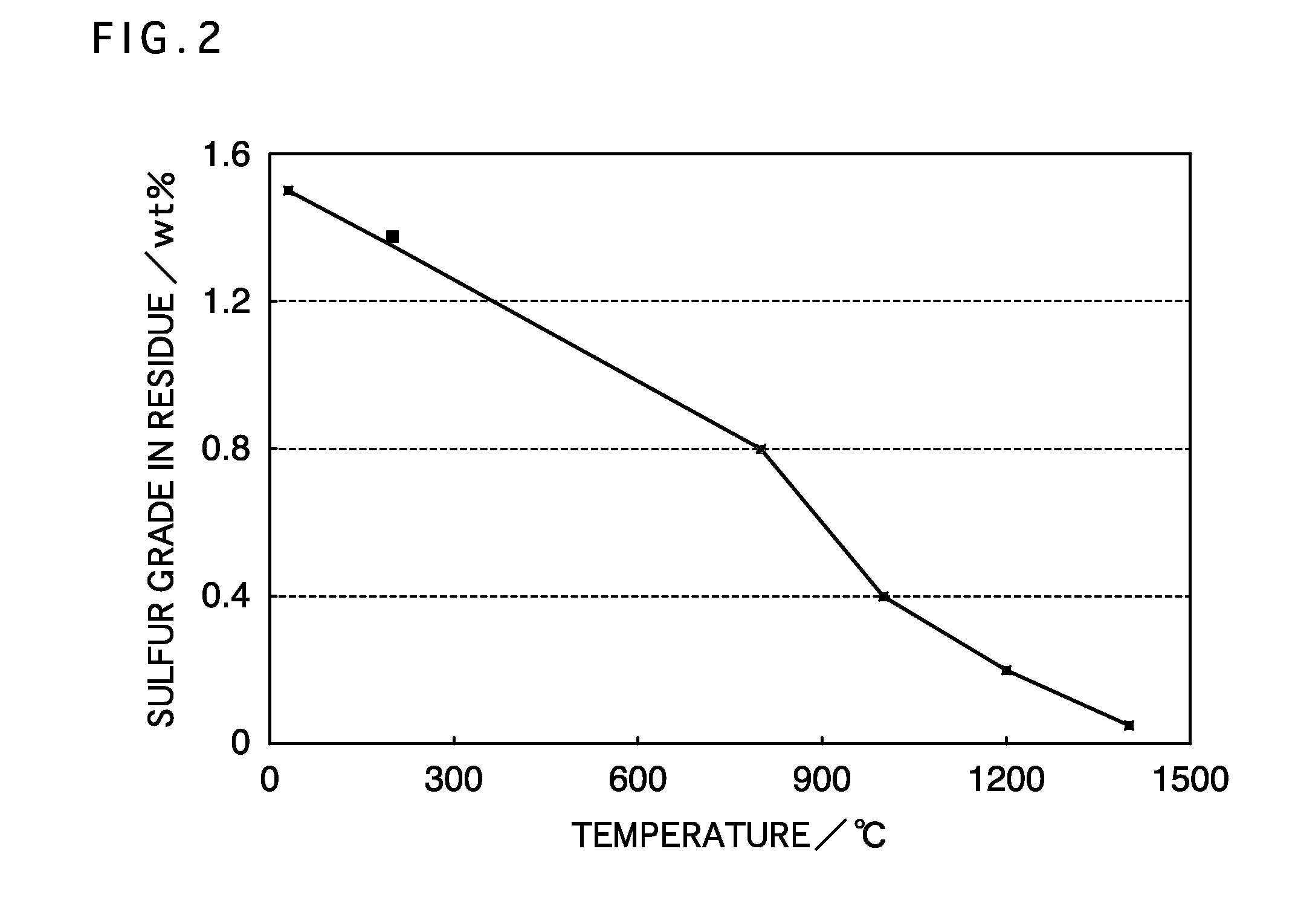
[0045]Next, the neutralized residue was equally divided into s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com