Supported Catalyst, Its Activated Form, and their Preparation and Use
a supported catalyst and activated form technology, applied in the field of porous catalysts, can solve the problems of raney nickel catalysts, inconvenient handling, and inability to use conventional fixed-bed reactions, and achieve the effects of low active metal loading amount, simple method, and low impurity conten
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Preparation of a Raney Ni / Polypropylene Catalyst
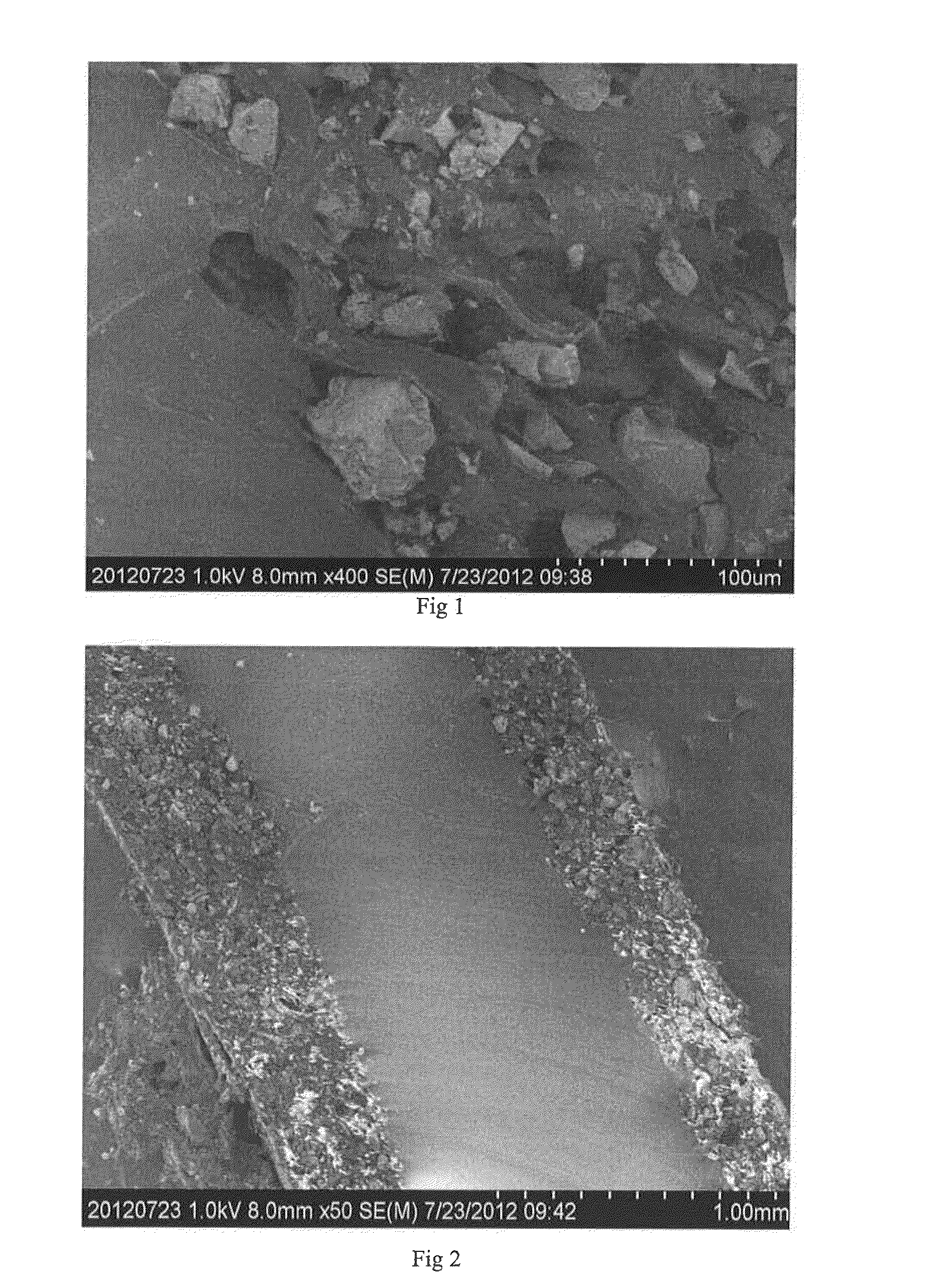
[0092]Powdery polypropylene (Maoming Petrochemical, F280M) was extruded with a twin screw extruder and cut into particles of Φ3 nm×3 to 5 mm. 50 Grams of the polypropylene particles were placed in 2000 g of 100 to 200 mesh powdery nickel-aluminum alloy having a Ni content of 48 wt. % and an aluminum content of 52 wt. %. The mixture of the polypropylene particles and the powdery nickel-aluminum alloy was mould pressed in a platen press at 200° C. under a pressure of 7 MPa for 10 min, then removed and cooled. The mixture was sieved to separate catalyst particles from the excessive powdery nickel-aluminum alloy, to obtain 200 g supported catalyst. It was visually observed that the surface of the catalyst particles was completely covered by the nickel-aluminum alloy powder.
[0093]400 g of 20 wt % NaOH aqueous solution was formulated with deionized water and added to 40 g of the above-prepared supported catalyst particles. The resultant ...
example 2
The Preparation of a Raney Ni / Polypropylene Catalyst
[0094]Powdery polypropylene (Maoming Petrochemical, F280M) was mould pressed in a platen press at 200° C. under a pressure of 7 MPa for 10 min, to form a sheet having a thickness of approximate 2 mm.
[0095]In the platen press, two layers of 100 mesh to 200 mesh powdery nickel-aluminum alloy each having a thickness of about 2 mm were spread above and below a polypropylene sheet weighing 20 g prepared above, respectively, with the nickel-aluminum alloy having a Ni content of 48 wt. % and an aluminum content of 52 wt. %. Mould pressing was carried out at a temperature of 200° C. under a pressure of 7 MPa for 10 min, then the polypropylene sheet was removed and cooled, to afford a supported catalyst sheet weighing 120 g. It was visually observed that both the upper and lower surfaces of the polypropylene sheet were covered completely by the nickel-aluminum alloy powder.
[0096]The catalyst sheet was mechanically cut into particles having ...
example 3
The Preparation of a Raney Ni / Nylon-6 Catalyst
[0098]50 Grams of Nylon-6 particles (Baling Petrochemical, BL2340-H, having a particle size of Φ2.5×3 mm) were placed in 2000 g of 100 to 200 mesh powdery nickel-aluminum alloy having a Ni content of 48 wt. % and an aluminum content of 52 wt. %. The mixture of the Nylon-6 particles and the powdery nickel-aluminum alloy was mould pressed in a platen press at 250° C. under a pressure of 7 MPa for 10 min, then removed and cooled. The mixture was sieved to separate catalyst particles from the excessive powdery nickel-aluminum alloy, to obtain 210 g supported catalyst. It was visually observed that the surface of the catalyst particles was completely covered by the nickel-aluminum alloy powder.
[0099]400 g of 20 wt % NaOH aqueous solution was formulated with deionized water and added to 40 g of the above-prepared supported catalyst particles. The resultant mixture was maintained at 85° C. for 4 hours, then the liquid was filtered off, and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com