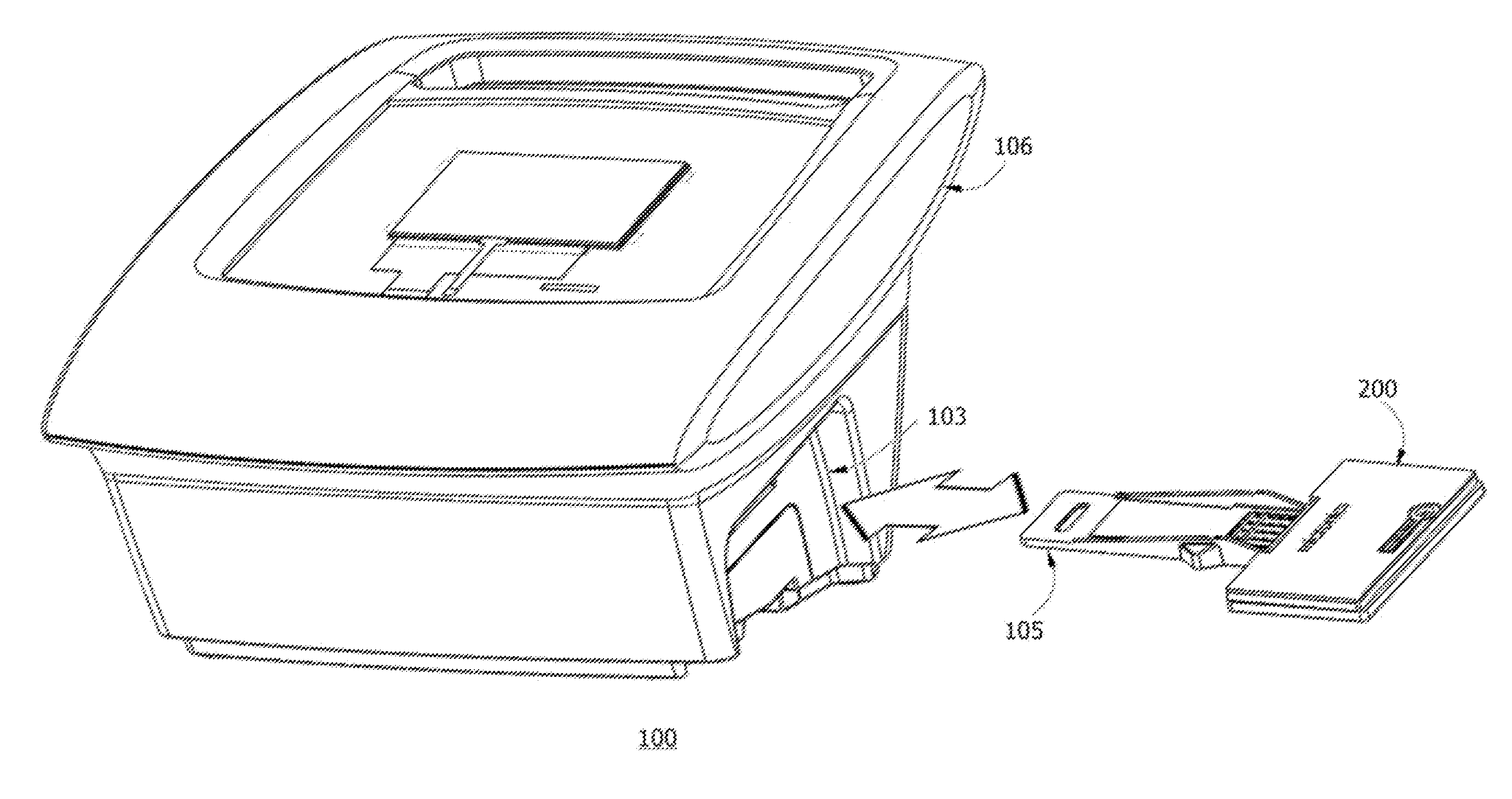
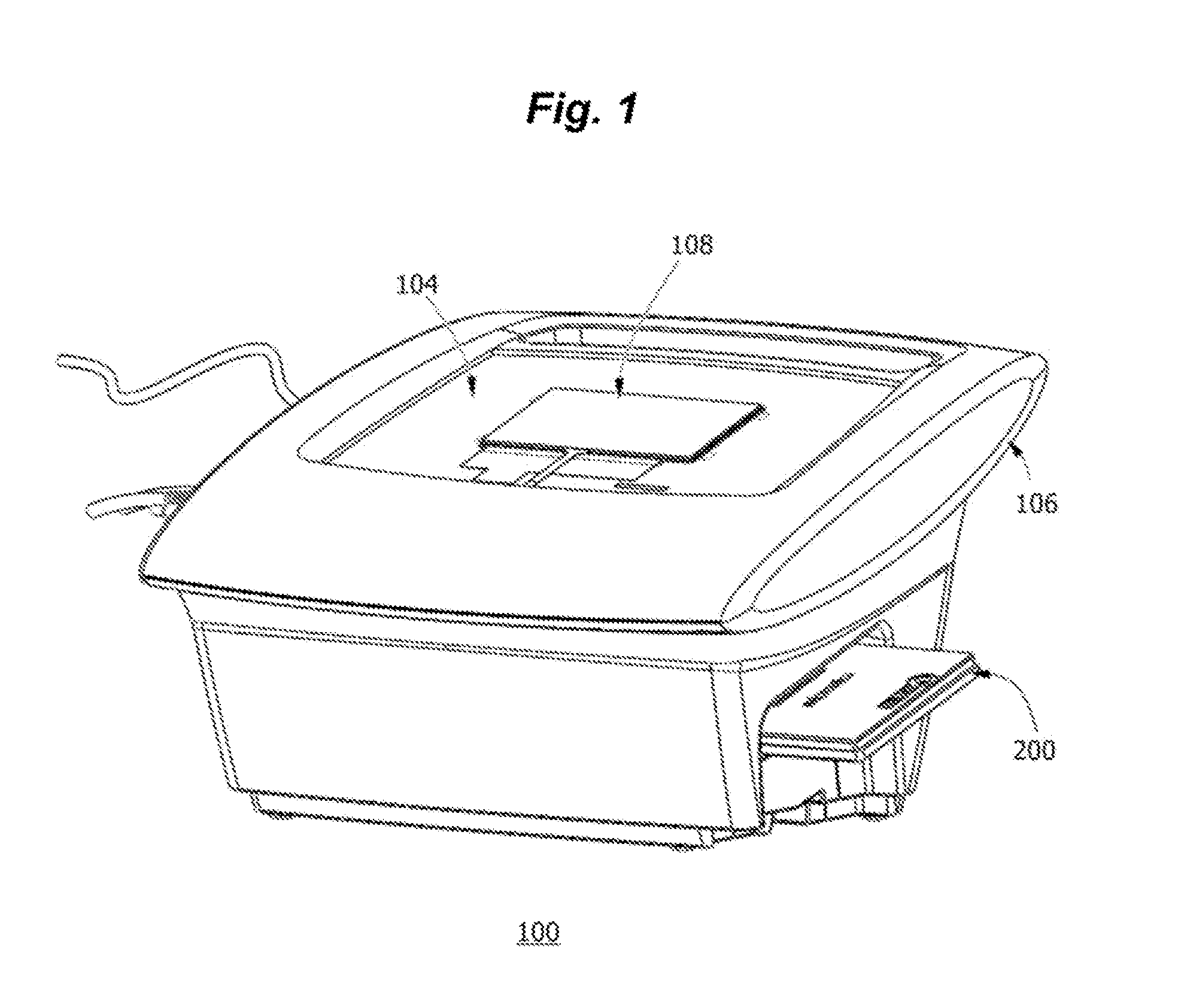
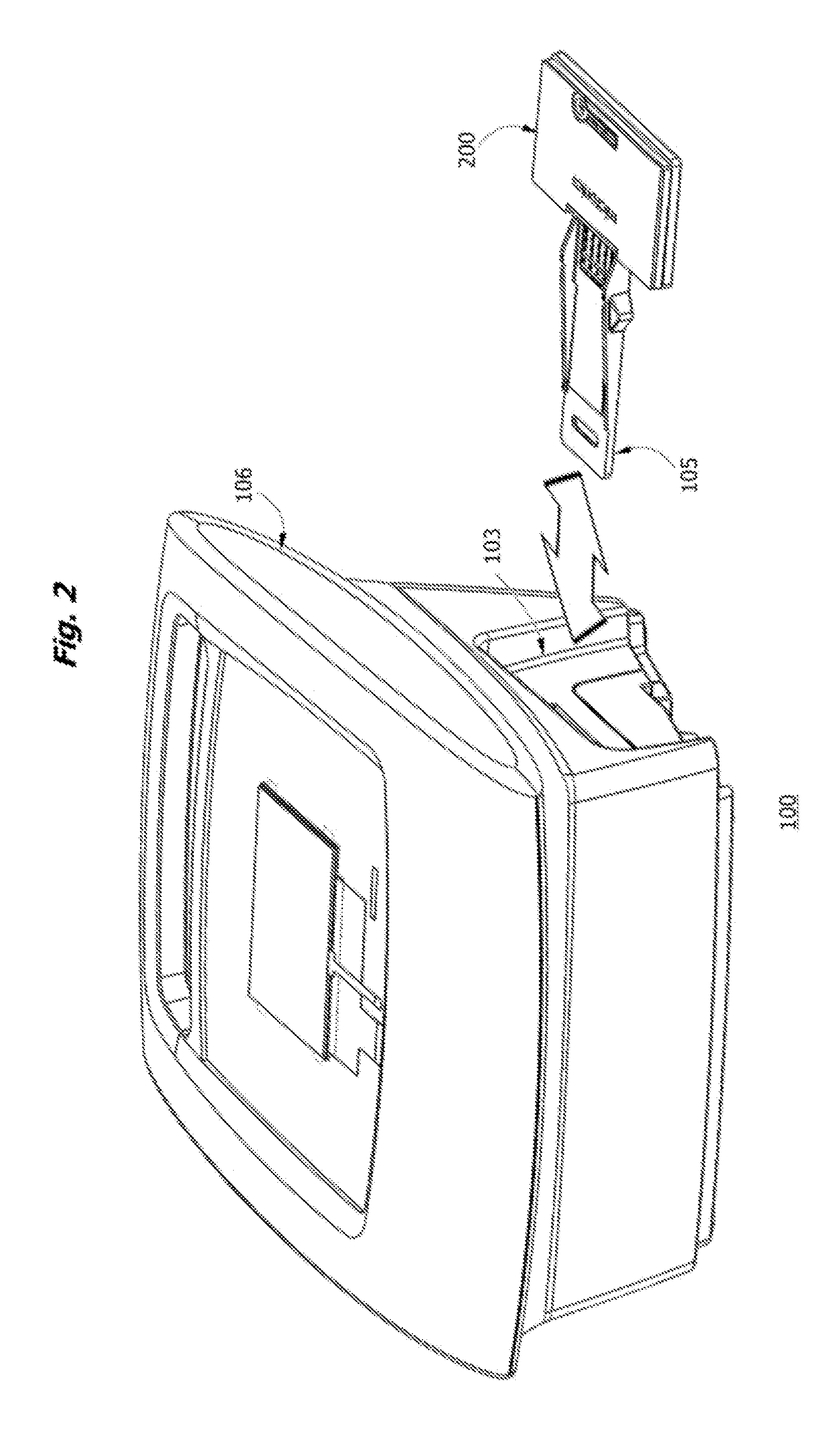
Portable fluorescence detection system and microassay cartridge
a fluorescence detection system and microassay cartridge technology, applied in the field of compact fluorescence detection instruments, can solve the problems of less effective conventional confocal localization of excitation and emission signals in generating robust signals over a wide range of sample and operating conditions, and achieve the effects of reducing the need for precision, improving the manufacturability of the apparatus, and high amplification gain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
[0207]In this example, the apparatus of the invention is shown to be useful in diagnosis of infectious disease by detection of the nucleic acids of a pathogen in a human sample such as blood. Using on-board dry and liquid reagents, a blood sample is processed and DNA associated with Plasmodium falciparum is detected in about 30 minutes or less. DNA purified from the sample is subjected to PCR using two chambers with dual temperature zones as described in U.S. Pat. Nos. 7,544,506, 7,763,453, and 7,955,836, which are co-assigned. Amplicon is then detected using a FAM fluorescence-tagged molecular beacon directed at the amplified target. Optionally, a control consisting of a California Red-tagged RNAase P leukocyte exon sequence, with multiplex amplification, is used to validate the assay. A representative thermal melt curve obtained using the thermo-optical interface of the invention is shown in FIG. 33B.
example ii
[0208]The apparatus of the invention is useful in the diagnosis of coagulopathies. Using on-board dry and liquid reagents, a blood sample is assayed for Coagulation Factor VIIa deficiency by incubating plasma with a fluorogenic substrate such as (D-Phe-Pro-Arg-ANSNH-cyclohexyl-2HC1; F.W.=777.81, Haematologic Technologies, Essex Junction Vt.) where ANSN is fluorophore 6-amino-1-naphthalene-sulfonamide, which lights up when the amide bond between the dye and the peptide is cleaved. Tissue Factor (TF) is obtained from Calbiochem (LaJolla Calif.) and incorporated into phosphatidylcholine or phosphatidylserine vesicles before use. TF is used in excess. A 100 uL substrate reaction mixture consisting of 20 mM Hepes buffer, pH 7.4, 0.15 M NaCl, with 5 nM TF and containing 20 uM EDTA is incubated with a plasma sample for 10 min to form the active enzyme complex. The ANSH substrate is then added. The rate of hydrolysis of substrate is linear over the normal range of Factor VIIa, and can be de...
PUM
| Property | Measurement | Unit |
|---|---|---|
| frequency | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com