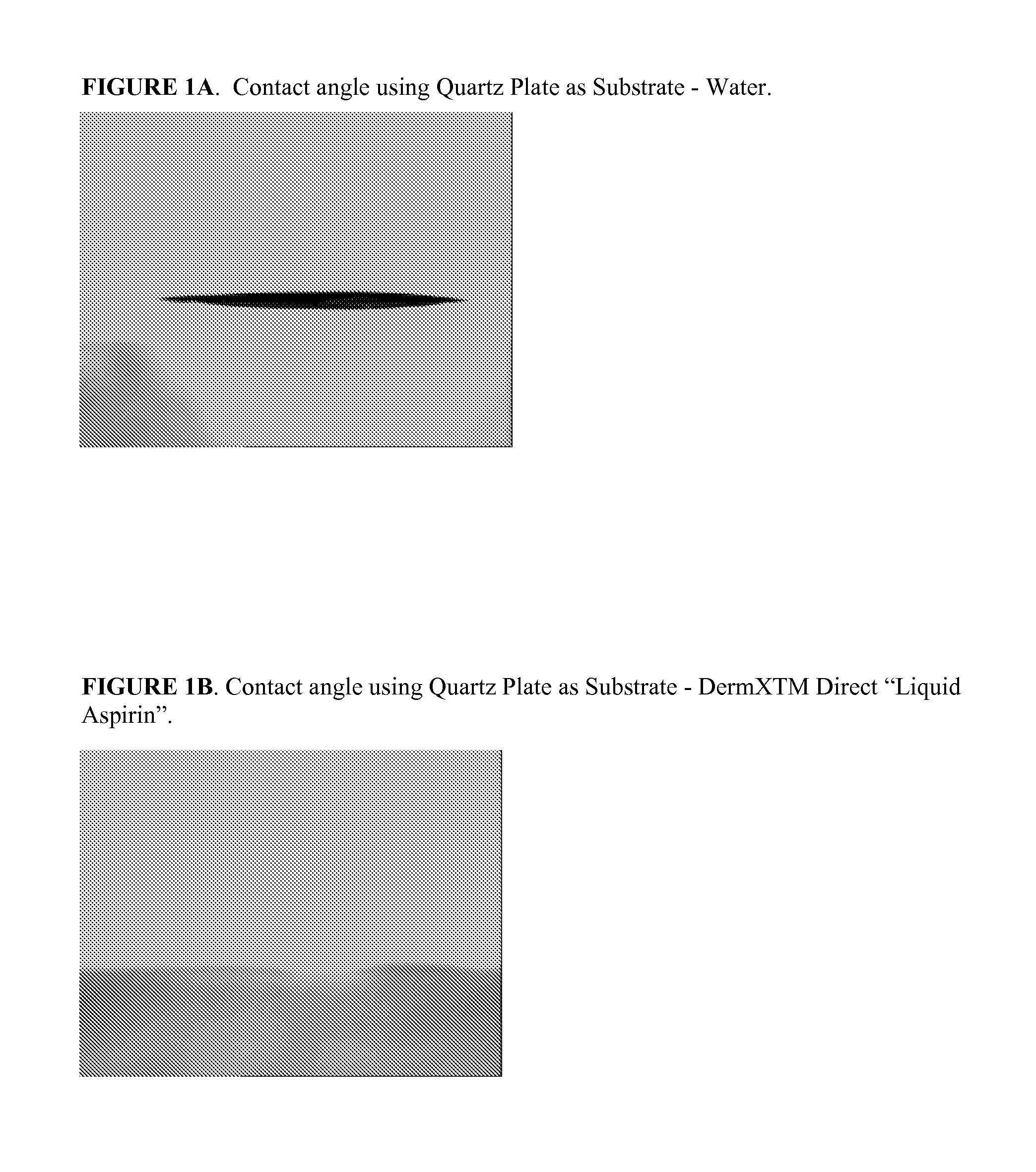
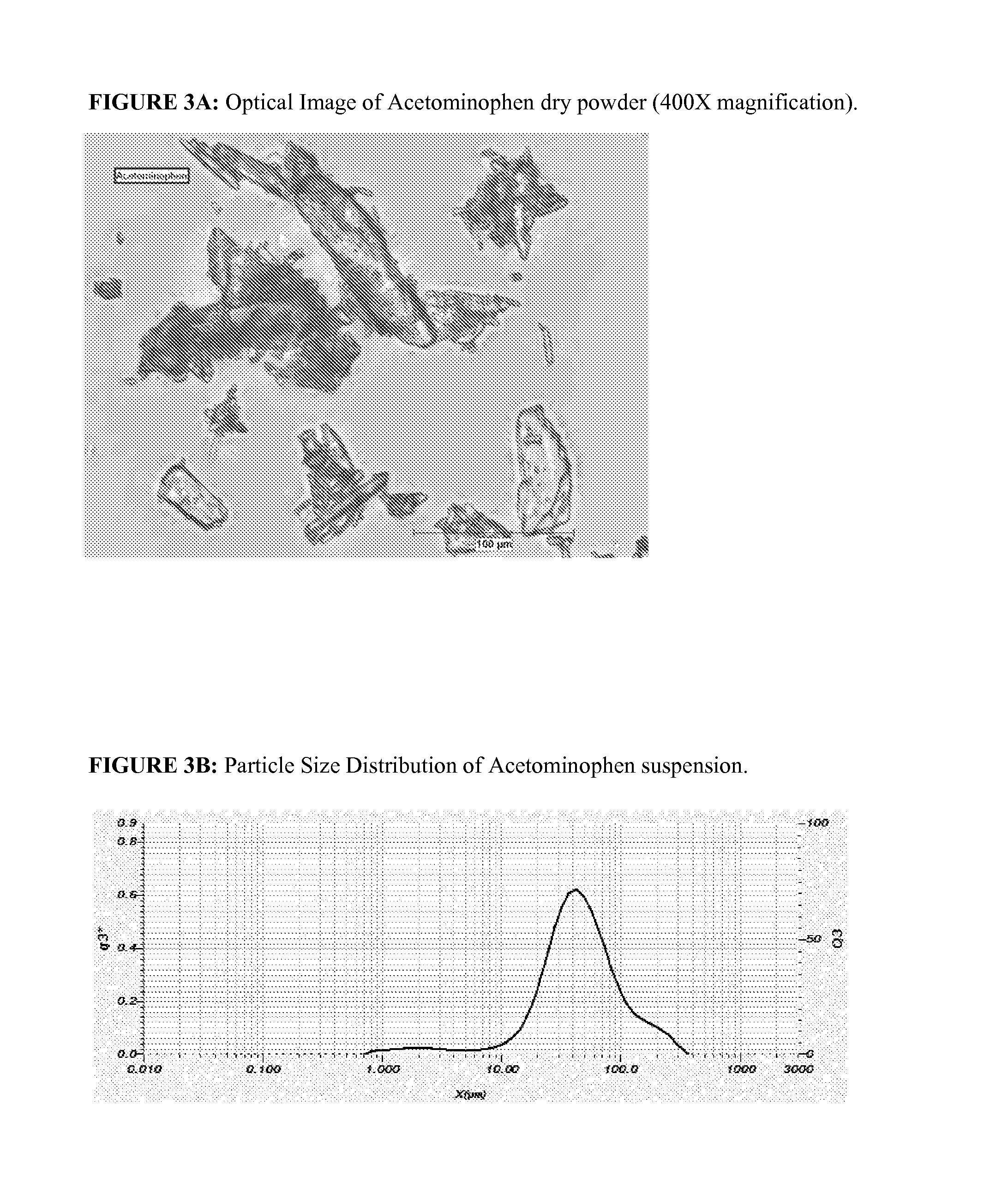
Liquid topical pharmaceutical nano-emulsion formulations
a technology of liquid and nanoemulsion, which is applied in the field of preparation, can solve the problems of increasing the risk of serious gastrointestinal adverse events, increasing the risk of serious cardiovascular thrombotic events, and myocardial infarction, and reducing the effect of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0298]Provided in this Example is an approach to prepare a transdermal and / or dermal composition (e.g., liquid) comprising an NSAID, acetaminophen, or insulin, wherein greater than 90% of the particles of the NSAID, acetaminophen, or insulin are less than or equal to 13 nanometers in size, as determined by a volume-weighted particle size distribution calculation. An exemplary formulation is shown below, but alternative formulations can be made by substituting another NSAID, acetaminophen, or insulin for aspirin (see below). Additionally, as noted above, the amounts of alcohol, active ingredient, and / or ethoxylated oil can be altered within the ranges provided in this disclosure so long as the amount of water added is adjusted to accommodate the increase or decrease in weight or volume afforded by the alteration in the amount of alcohol, active ingredient, and / or ethoxylated oil.
[0299]Liquid Aspirin 2.2% Formulation (Quantity=100 g, which is approximately 100 mL).
[0300]Perform the fo...
example 2
[0319]Additional NSAID transdermal and / or dermal compositions (e.g., liquids) can be readily prepared following the approach described in Example 1 with the following modifications.
[0320]Diclofenac Sodium 2.2% formulation: Substitute 2.2% Diclofenac Sodium for 2.2% aspirin.
[0321]Diclofenac Epolamine 2.2% formulation: Substitute 2.2% Diclofenac Epolamine for 2.2% aspirin.
[0322]Salicylic Acid 2.2% formulation: Substitute 2.2% Salicylic Acid for 2.2% aspirin.
[0323]Indomethicin 2.2% formulation: Substitute 2.2% Indomethicin for 2.2% aspirin.
[0324]Etodolac 2.2% formulation: Substitute 2.2% Etodolac for 2.2% aspirin.
[0325]Ketorolac 2.2% formulation: Substitute 2.2% Ketorolac for 2.2% aspirin.
[0326]Meloxicam 2.2% formulation: Substitute 2.2% Meloxicam for 2.2% aspirin.
[0327]Piroxicam 2.2% formulation: Substitute 2.2% Piroxicam for 2.2% aspirin.
[0328]Nabumetone 2.2% formulation: Substitute 2.2% Nabumetone for 2.2% aspirin.
[0329]For transdermal and / or dermal compositions (e.g., liquids) with...
example 3
[0330]A prior art dermal and / or transdermal composition (liquid) for use in comparative particle size and stability studies is prepared as follows:
[0331]Liquid Aspirin 2.2% (Prior art) Formulation (Quantity=100 g, which is approximately 100 mL).
[0332]Perform the following steps in order:
[0333]1. Bring 2.2±0.1 g USP grade aspirin into solution with 37.7 ml (approximately 38%) of distilled or deonized water.
[0334]2. Add 50 g of 200 Proof Ethyl Alcohol (approximately 50%) mixing continuously with a stir bar.
[0335]3. Slowly add 10 g (approximately 10%) of macadamia nut oil having 16 ethoxylations per molecule while mixing.
[0336]4. Once the emulsion has formed, add 0.13 g Peace & Calming Oil (fragrance dropwise.
[0337]5. Continue stirring until bottling.
[0338]6. Optionally, a cream, rinse, hydrogel, serum, gel, lotion, paste or puddy can be obtained by adding an appropriate amount of one or more viscosity-increasing agents such as, a cellulose derivative that is selected from the group co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com