Low Emissions Polyurethane Foam Made with Isocyanate Reactive Amine Catalyst
a reactive amine and polyurethane foam technology, applied in the field of tertiary amine catalysts, can solve the problems of poor dimensional stability, large shrinkage, poor physical properties, etc., and achieve the effect of reducing amine emissions and avoiding the exposure of end users to amines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of N,N-Bis-(dimethylaminopropyl)-N-(3-aminopropyl)-amine (Amine-1)
[0065]In the first step, a 1000 ml stainless steel reactor was charged with 424 g of bis(dimethylaminopropyl) amine and 23 g of water. The reactor was purged with nitrogen, heated up to 75° C. and 126 g of acrylonitrile was slowly fed in the reactor over a period of 1.5 hours. After all acrylonitrile was transferred into the reactor the temperature was maintained at 75° C. for an additional 4.0 hours. The reaction mixture was allowed to cool down to 25° C. and the product was removed from the reactor and analyzed by GC giving 96% yield of desired product 2-cyanoethyl-bis(dimethylaminopropyl)amine. In the second step, a 1000 ml stainless steel reactor was charged with 198 g of isopropanol and 6.9 g of standard Raney-Cobalt catalyst. The reactor was purged with nitrogen three times and the temperature was increased to 120° C. The reactor was pressurized with 800 psi of hydrogen and cyanoethyl-bis(dimethylamino...
example 2
Physical Properties of PU Foam Made with Various Gelling Catalysts and Their Comparison with Standard Catalysts Dimethylaminopropyl Urea, and Bis(dimethylaminopropyl) Urea
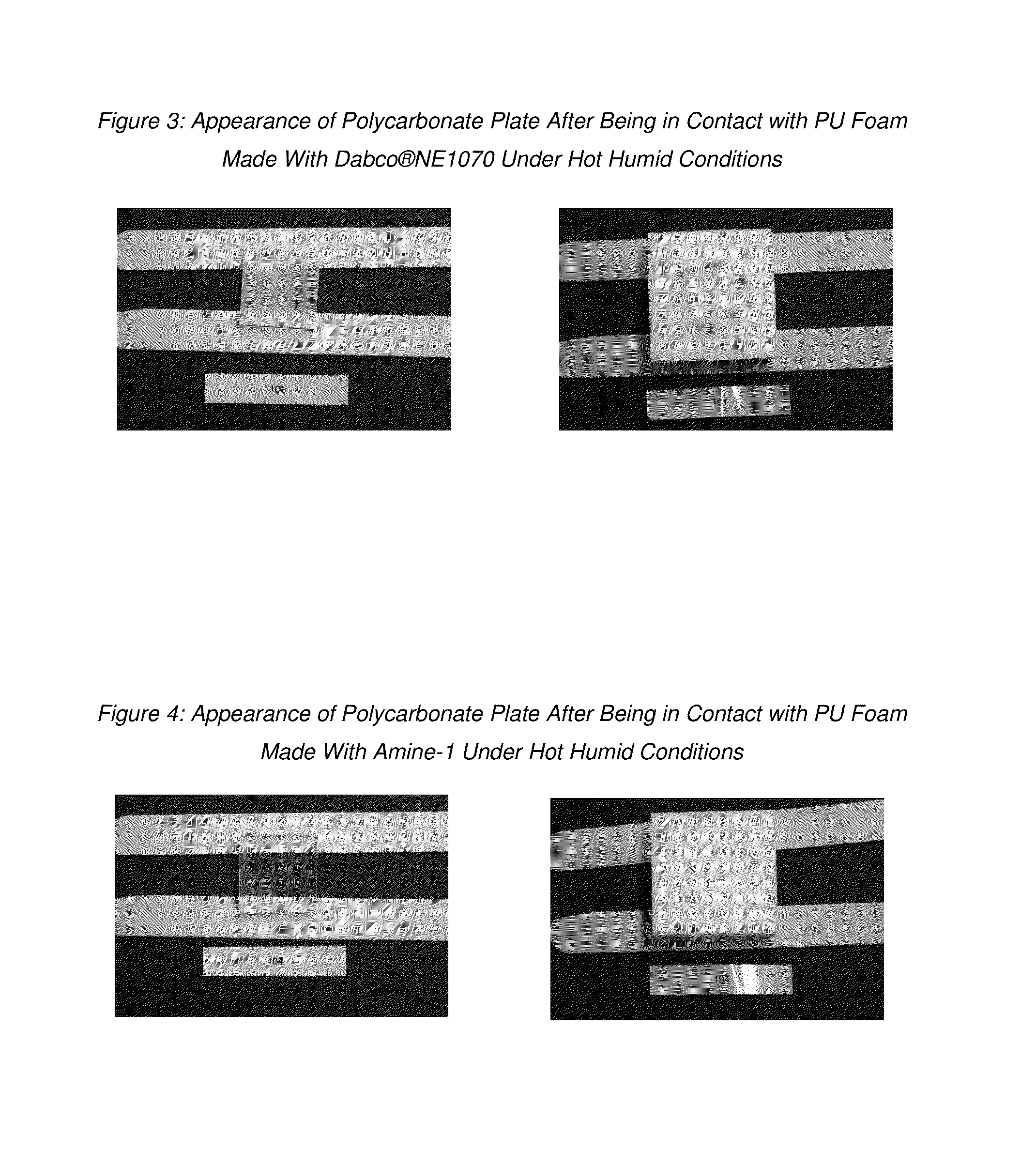
[0066]Foam pads were prepared by adding a tertiary amine catalyst to about 302 g of a premix (prepared according to the formulation shown in Table II) in a 32 oz (951 ml) paper cup. The formulation was mixed for about 10 seconds at about 6,000 RPM using an overhead stirrer fitted with a 2-inch (5.1 cm) diameter stirring paddle. Toluene diisocyanate (TDI) was then added, and the formulation was mixed well for about another 6 seconds at about 6,000 RPM using the same stirrer, after which it was poured into a pre-heated mold at 70° C. and demolded after 4 minutes. The foam pads were removed from the mold, hand crushed, weighed and machine crushed at 75% pad thickness. Foam pads were stored under constant temperature and humidity conditions for 48 hours before being cut and tested.
TABLE IIFORMULATION COMPONENTSComponen...
example 3
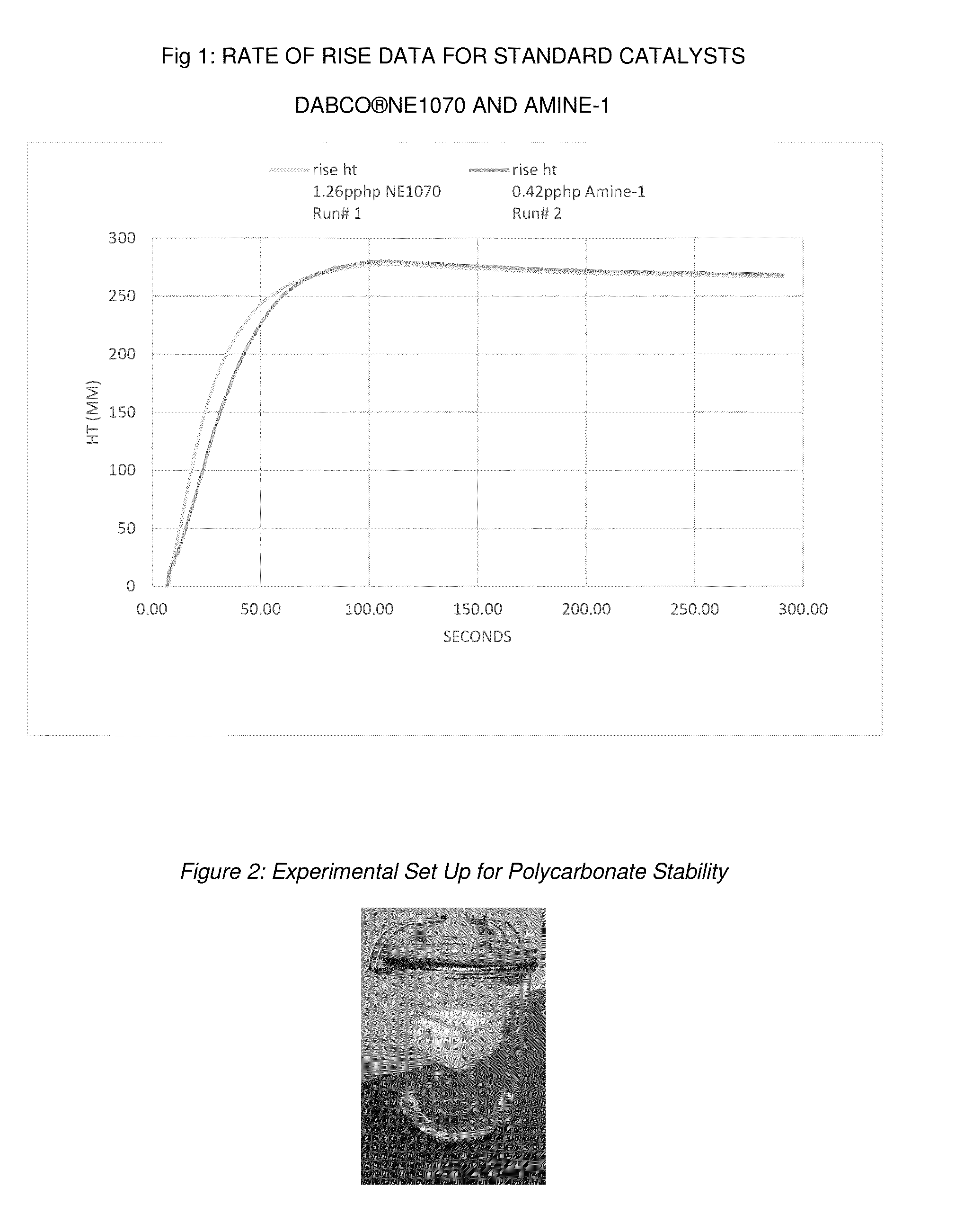
[0069]Foam Rate of Rise Kinetics and Use Level Comparison for N,N-Bis-(dimethylaminopropyl)-N-(3-aminopropyl)-amine
[0070]Foaming performance can be evaluated by comparing the foam height versus time for standards and new amine catalyst. Foam height profile can be measured by automated rate of rise equipment, utilizing free-rise cup foam samples with a FOMAT sonar rate-of-rise device (hereafter referred to as a “ROR”). The FOMAT device comprises a sonar sensor that measures and records the height in millimeters (mm) of the rising foam sample versus time in seconds (s), directly after mixing all components of the formulation. The FOMAT standard software generates both height versus time plots and velocity versus time plots. These plots are useful for comparing the relative reactivity of different catalyst formulations. Flexible open celled foam can be prepared by combining a total weight of about 300 g of the ingredients in Table II other than the isocyanate in a 32-oz (951 ml) paper ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperatures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com