Methods of preparing Anti-human papillomavirus antigen t cells
a technology of human papillomavirus and antigen t cells, which is applied in the field of preparation of antihuman papillomavirus antigen t cells, can solve the problems of poor prognosis of many cancers, including hpv-associated cancers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0085]This example demonstrates a method of preparing HPV-positive tumor-infiltrating lymphocytes (TIL) for adoptive cell therapy.
[0086]Patients were entered into clinical protocols and signed informed consents that were approved by the Institutional Review Board of the National Cancer Institute prior to tumor resection. Tumors were excised from patients. Tumors were tested for HPV 16 E6, HPV 16 E7, HPV 18 E6, and HPV 18 E7 expression using reverse transcriptase (RT) polymerase chain reaction (PCR) genotyping.
[0087]Multiple (24) independent cultures of HPV 16 E6 positive, HPV E7 positive, HPV 18 E6 positive, and HPV E7 positive TIL were set up using enzymatic digests and tumor fragments (1-2 mm3) procured by sharp dissection. TIL from tumor digests were generated by culturing single-cell suspensions (5×105 / mL) obtained by overnight enzymatic digestion of tumor fragments in media containing collagenase, hyaluronidase, and DNAse. Cultures of tumor fragments and digests were initiated ...
example 2
[0091]This example demonstrates the reactivity of the TIL from Patient 1.
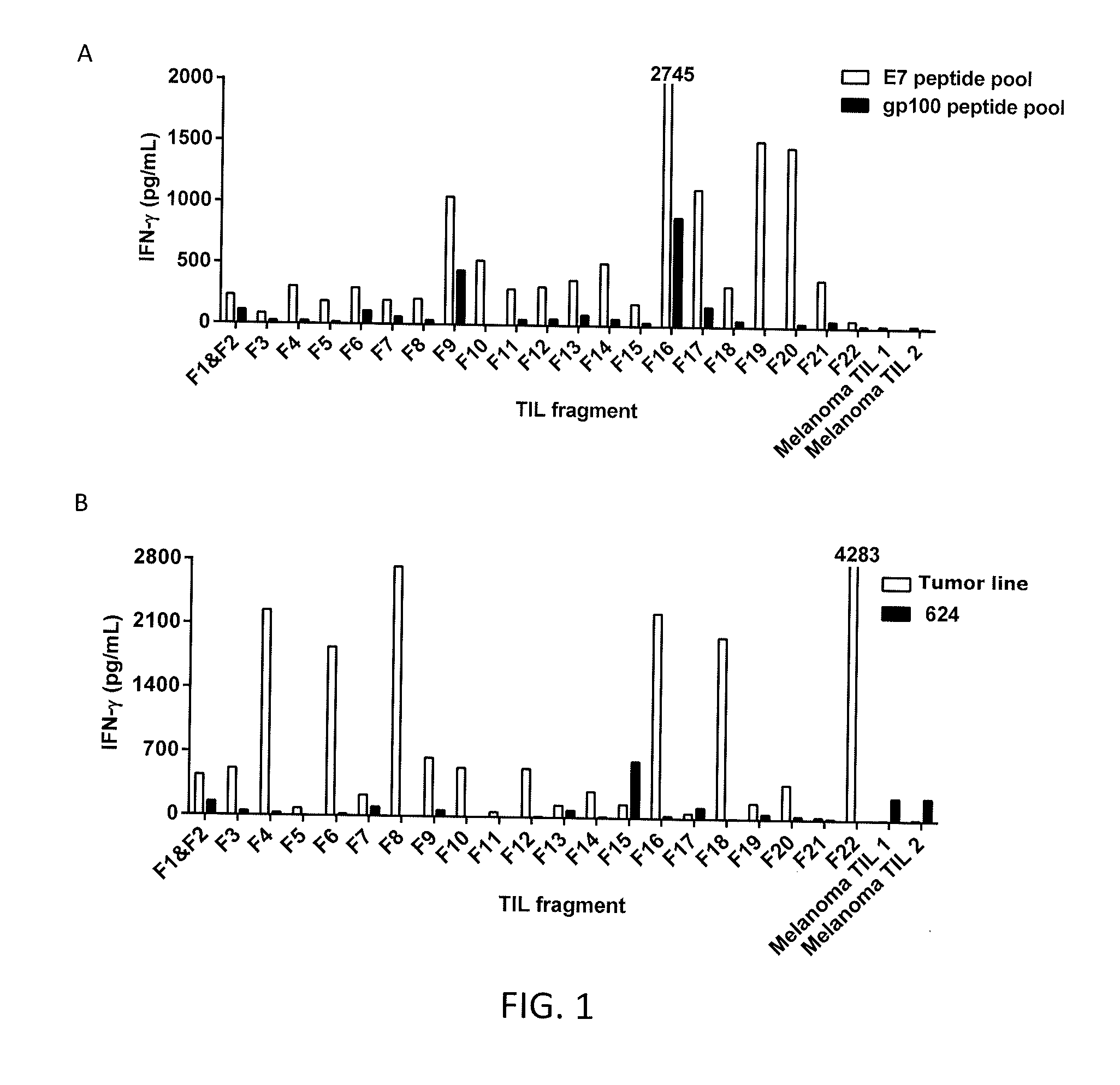
[0092]TIL were generated as described in Example 1 from 22 different tumor fragments (F1-F22) from Patient 1. The TIL from Patient 1 or melanoma TIL (control) were co-cultured with dendritic cells pulsed with the HPV 18 E7 peptide pool or a gp100 peptide pool (control) and IFN-γ was measured. The results are shown in FIGS. 1A-1B. As shown in FIGS. 1A-1B, the TIL from tumor fragment 22 of Patient 1 recognized an autologous tumor line but not HPV 18 E7 peptides.
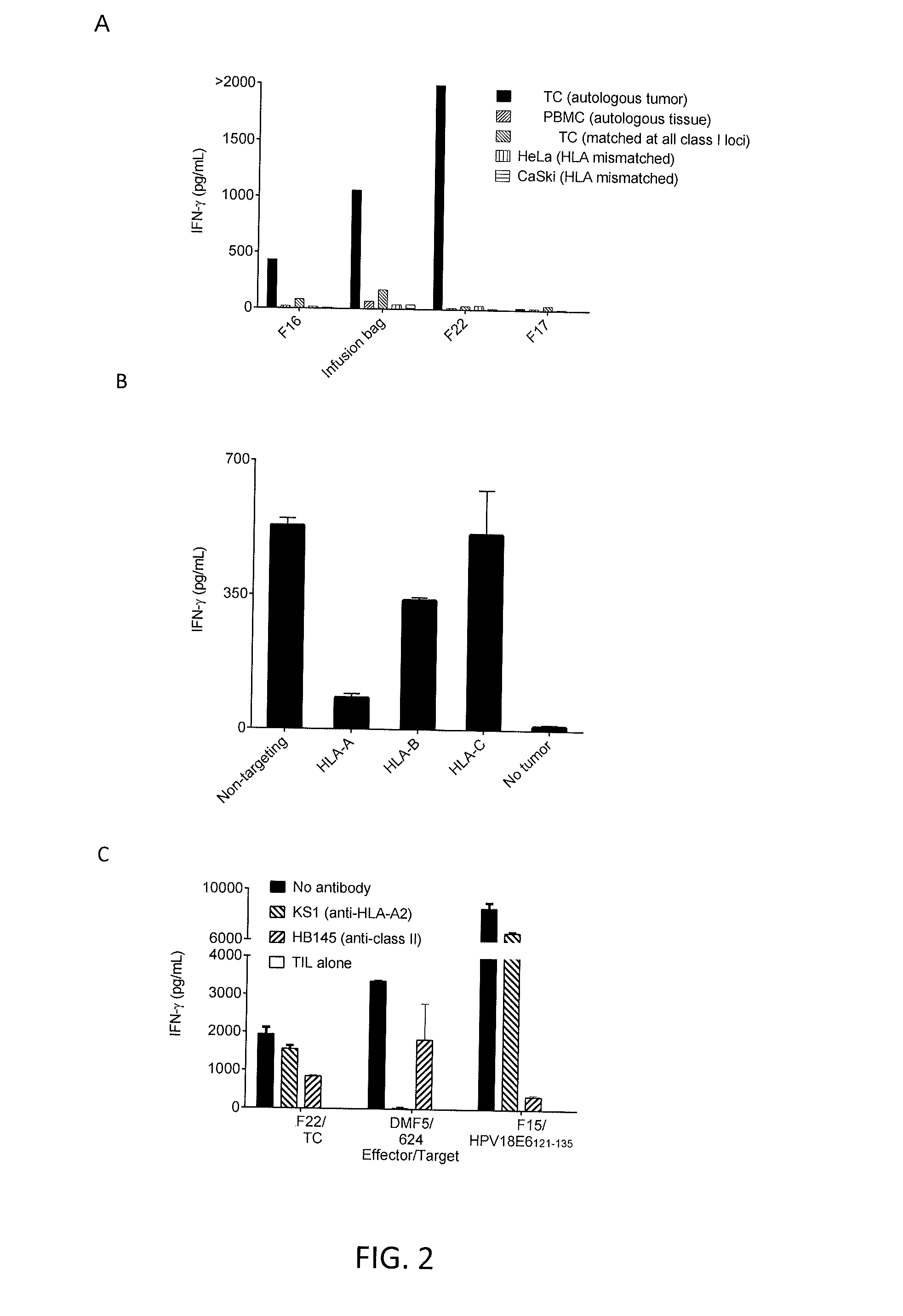
[0093]The TIL from tumor fragments F16, F17, or F22 of Patient 1 or cells given to the patient for treatment (“infusion bag”) were co-cultured with autologous tumor, peripheral blood mononuclear cells (PBMC) from autologous tissue, tumor cells matched at all class I loci, HeLa cells (HLA mismatched), or CaSki cells (HLA mismatched). IFN-γ was measured. The results are shown in FIG. 2A. As shown in FIG. 2A, TIL from tumor fragments F16 and F22 showed autolo...
example 3
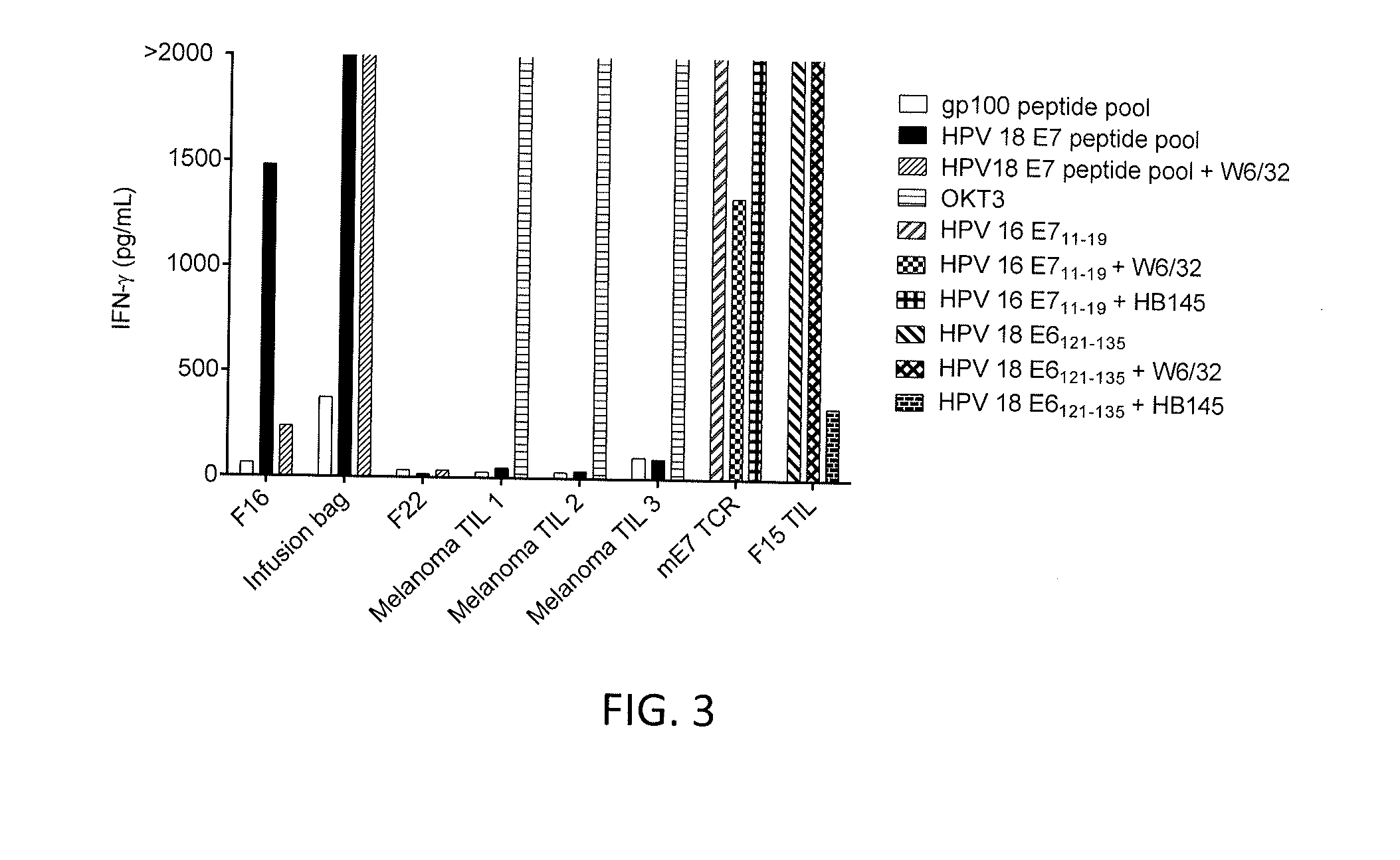
[0097]This example demonstrates the cloning of TIL from tumor fragment 16 of Patient 1 to isolate HPV 18 E7 reactive CD8 positive T cells.
[0098]DCs were loaded with HPV 18 E7 and co-cultured with TIL from tumor fragment 16 (F16) of Patient 1. The TIL were sorted for 4-1BB positive cells using fluorescence activated cell sorting (FACS). The sorted cells were cultured in 96-well plates with two cells per well. The clones were screened for tumor reactivity against a gp100 peptide pool or a HPV 18 E7 peptide pool. The results are shown in FIGS. 4A and 4B. As shown in FIGS. 4A and 4B, CD8 positive T cell cloning from tumor fragment F16 using 4-1BB-based FACS sorting resulted in the isolation of two clones (12 and 21) with E7 peptide pool reactivity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com