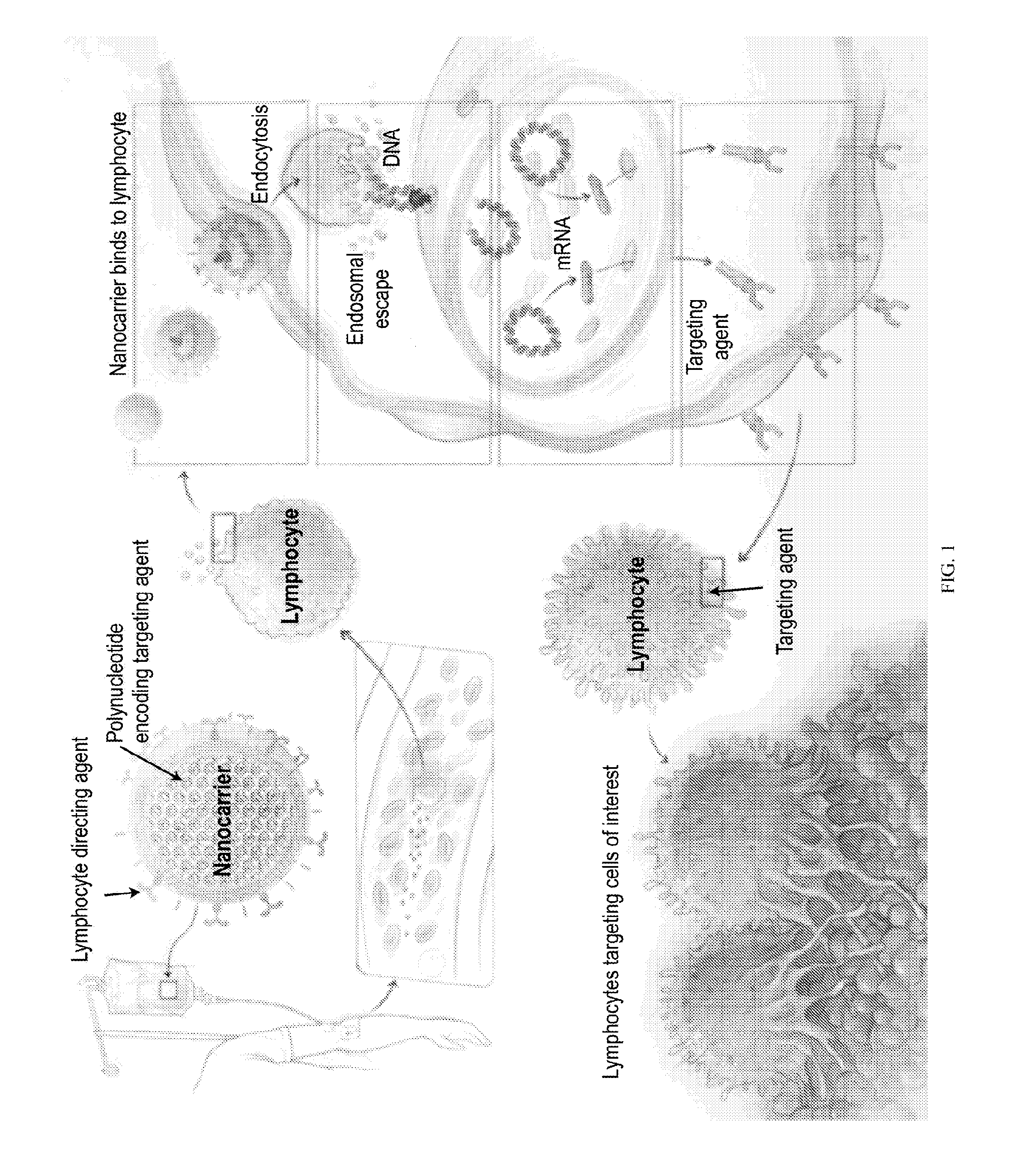
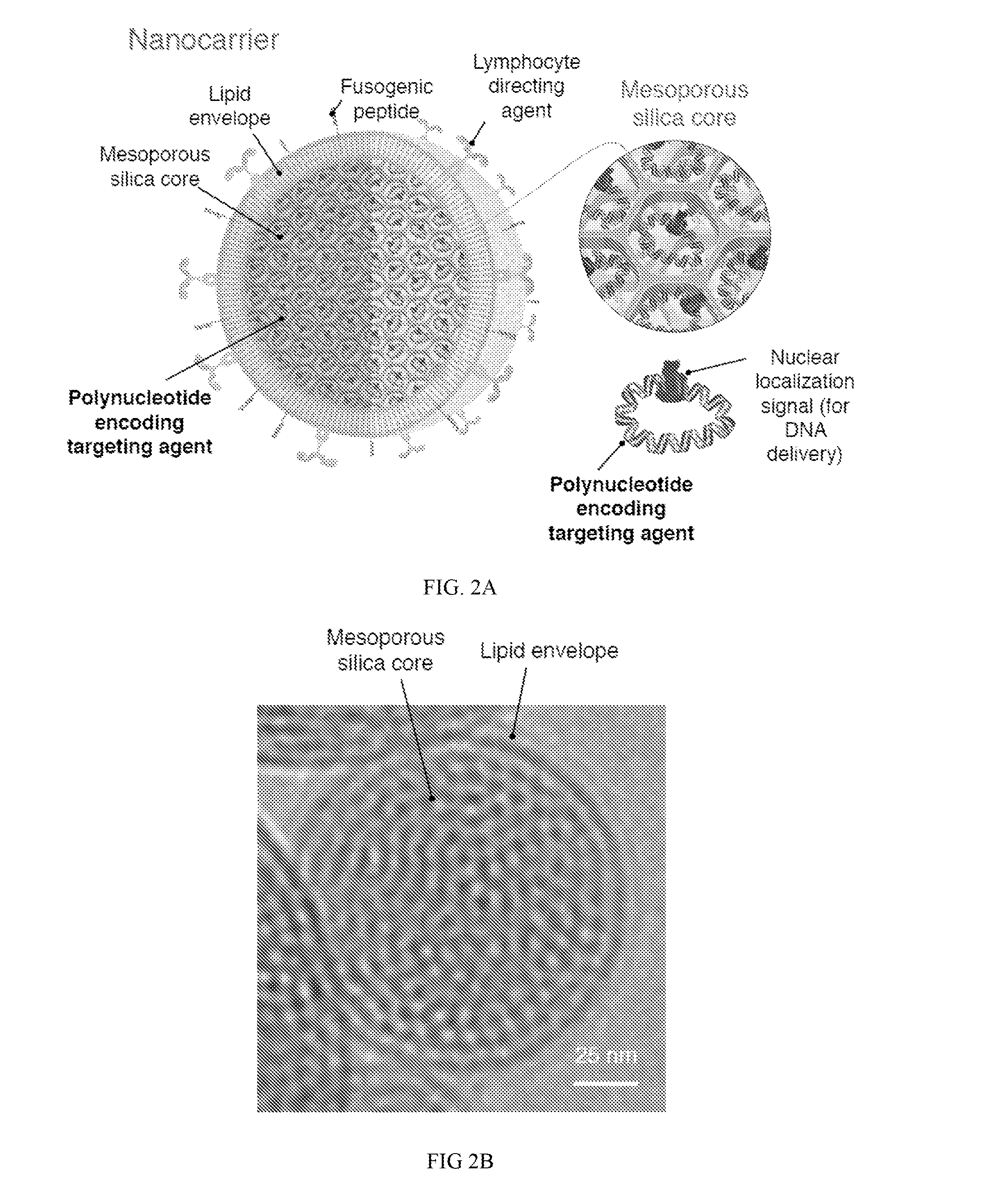
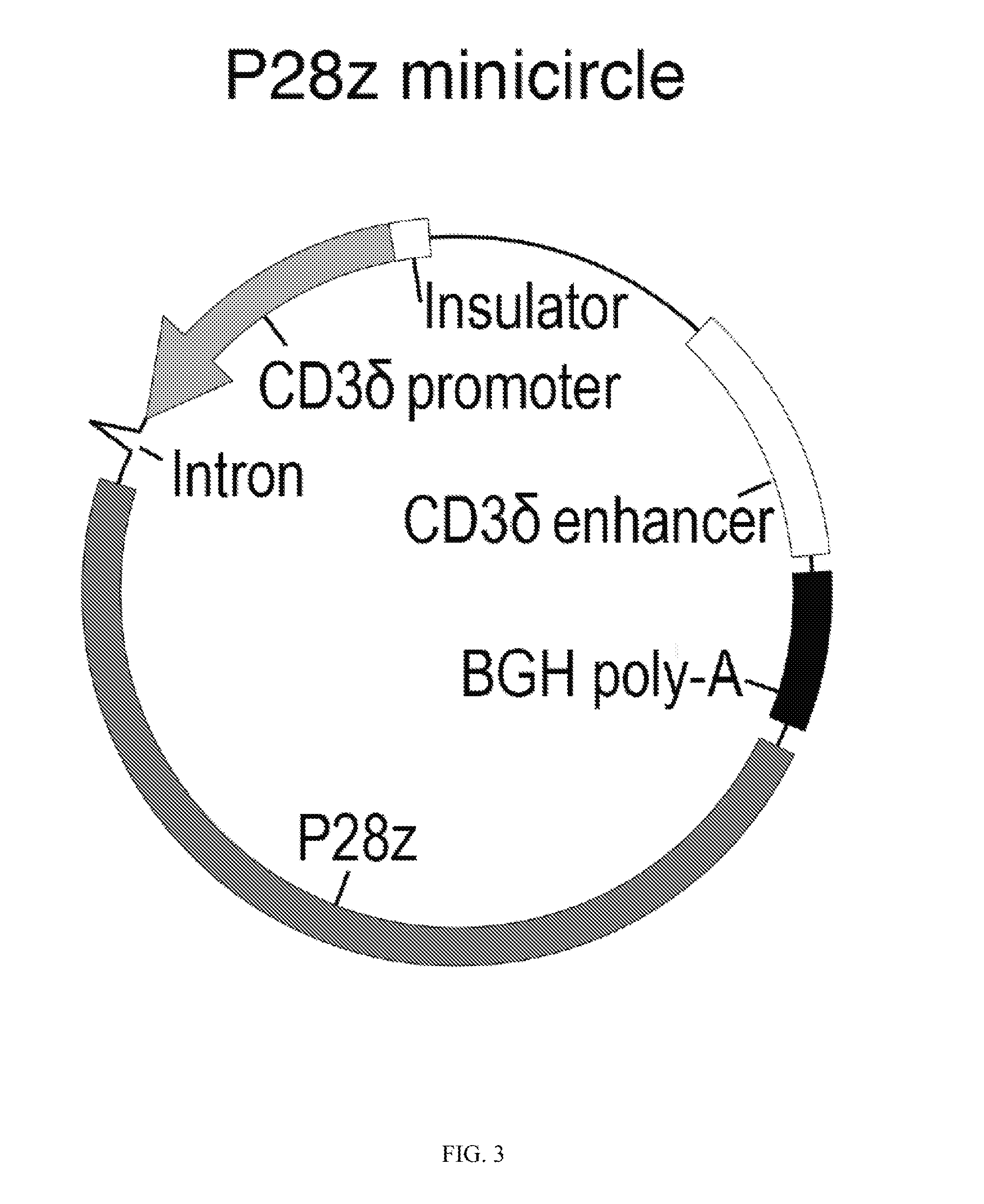
Compositions and methods to modify cells for therapeutic objectives
a technology of cells and molecules, applied in the field of compositions and methods to modify cells for therapeutic objectives, can solve the problems of ineffective anti-managed cells, depletion of immune system resources, and ineffective conventional vaccine approaches against cancer cells and cells affected by certain infectious diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0287]This example demonstrates that synthetic nanoparticles containing TCR genes can be used to generate functional tumor- or virus-specific T-cells. Lipid nanoparticles (FIG. 2A) were loaded with a minicircle gene (FIG. 3) encoding the chimeric antigen receptor P28z. P28z is a fusion receptor composed of a single-chain antibody (scFv) specific for the extracellular domain of PSMA (J591) combined with CD28 and CD3 cytoplasmic signaling domains (FIG. 4A; SEQ ID NO. 94). In this Example, chimeric antigen receptors (CARs) are fusion receptors including an antigen-binding domain, a transmembrane domain and an intracellular signaling domain resulting in T-cell activation after antigen binding. The P28z CAR directs T-cells toward the prostate-specific membrane antigen (PSMA), which is highly expressed on prostate cancer cells. Therefore, the introduction of the P28z gene into T-cells renders them capable of recognizing and lysing prostate tumor. The P28z gene was cloned under the control...
example 2
[0291]CD3-targeted protocell nanoparticles selectively bind circulating T cells in mice. A goal of the current disclosure is to selectively and quickly edit lymphocyte specificity in vivo to target unwanted cells. To examine how selectively protocells bind circulating host T cells, mice were systemically injected with 1×1011 fluorescently tagged nanoparticles. After 6 hours peripheral blood was collected by retro-orbital puncture and the percentage of fluorescent T cells was quantified by flow cytometry. CD3-targeted protocells labeled the majority of T cells in the blood, with relatively low binding to off-target cells (FIG. 4E, left panel). Confocal imaging of sorted T cells showed that nanoparticles are rapidly internalized from the cell surface into the cytoplasm as a result of receptor-induced endocytosis (FIG. 4E, right panel).
example 3
[0292]Generating an orthotopic bioluminescent mouse model for analyzing treatment of metastatic prostate cancer. Male TRAMP transgenic mice spontaneously develop orthotopic prostate tumors following puberty. However, unlike human prostate adenocarcinoma, TRAMP tumors do not express significant amounts of PSMA, a target in experiments using the P28z CAR. Furthermore, longitudinal studies to measure the prostate cancer volume in TRAMP animals rely on expensive and time-consuming magnetic resonance imaging (MRI) techniques, which preclude analysis of large cohorts of mice. To overcome these issues, a cell line from a primary TRAMP tumor was established and the PSMA gene was introduced through retroviral transduction. To serially monitor tumor burden by bioluminescence imaging, tumor cells were also genetically tagged with Firefly luciferase (FLuc). Following orthotopic transplantation into the dorsal lobe of the prostate gland of C57BL / 6 mice, TRAMP-PSMA-FLuc tumor cells reproducibly d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| mean diameter | aaaaa | aaaaa |
| mean diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com