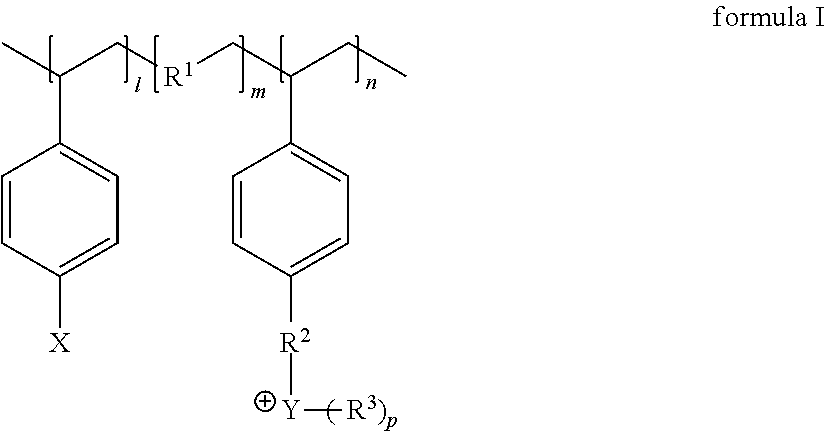
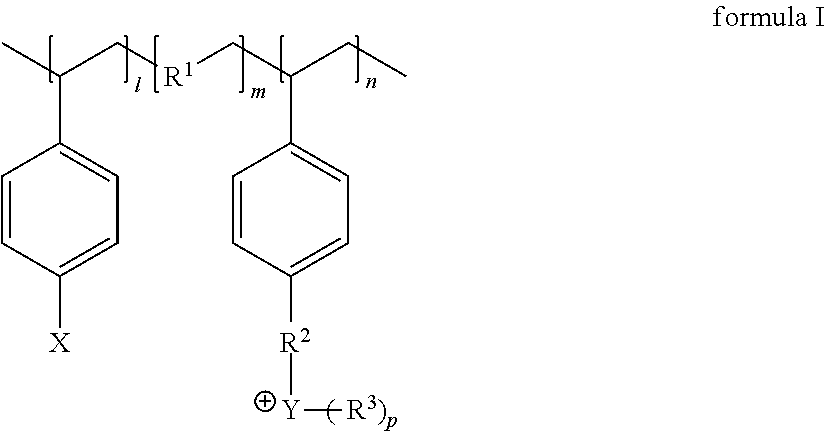
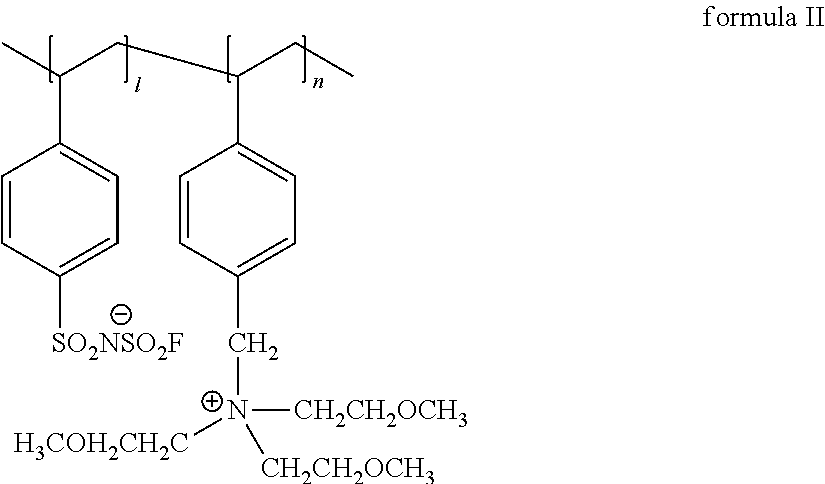
Polymer protecting layer, lithium metal negative electrode, lithium secondary battery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0049]Preparation of the positive electrode P1#: lithium cobalt oxide (LiCoO2, positive active material), conductive carbon black (Super-P, conductive agent) and polyvinylidene fluoride (PVDF, binder) were uniformly dispersed in 1-methyl-2-pyrrolidinone (NMP, solvent) to form a positive electrode slurry. A solid content of the positive electrode slurry was 77 wt %, solid components in the positive electrode slurry were 98.26 wt % of lithium cobalt oxide, 0.9 wt % of PVDF and 0.84 wt % of conductive carbon black. Then the positive electrode slurry was uniformly coated on two surfaces of an aluminum foil (positive current collector) with a thickness of 12 μm, a coating weight on each surface of the aluminum foil was 0.0215 g / cm2; then a drying process was performed at 85° C., which was followed by cutting into a disk with a diameter (Φ) of 14 mm, and then the disk was dried under vacuum at 85° C. for 4 h, the obtained positive electrode was marked as P1#.
[0050]Preparation of the polym...
example 2
[0054]Preparation of the lithium metal negative electrode N2#: 0.8 g of Al2O3 was added into 8 g of 1-methyl-2-pyrrolidinone (NMP, solvent) by means of ultrasonic dispersion for 30 min, then 1 g of polymer ionic liquid L1# (formula II) and 0.2 g of polyvinylidene fluoride (PVDF, the number-average molecular weight was about 1,000,000) were added and stirred constantly for 5 h, the obtained mixture was uniformly coated on two surfaces of the lithium metal foil, a lithium metal negative electrode was obtained, the obtained lithium metal negative electrode was marked as N2#.
[0055]Preparation of the lithium secondary battery C2#: the preparation of the lithium secondary battery was the same as the preparation of the lithium secondary battery C1#, except that the used lithium metal negative electrode was N2#, and the obtained lithium secondary battery was marked as C2#.
example 3
[0056]Preparation of the lithium metal negative electrode N3#: 0.8 g of Al2O3 was added into 8 g of 1-methyl-2-pyrrolidinone (NMP, solvent) by means of ultrasonic dispersion for 30 min, then 1 g of polymer ionic liquid L1# (formula II) and 0.2 g of polyethylene oxide (PEO, the number-average molecular weight was about 600,000) were added and stirred constantly for 5 h, the obtained mixture was uniformly coated on two surfaces of the lithium metal foil, a lithium metal negative electrode was obtained, the obtained lithium metal negative electrode was marked as N3#.
[0057]Preparation of the lithium secondary battery C3#: the preparation of the lithium secondary battery was the same as the preparation of the lithium secondary battery C1#, except that the used lithium metal negative electrode was N3#, and the obtained lithium secondary battery was marked as C3#.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com