Mutagenic nucleoside analogs and uses thereof
a technology of nucleoside analogs and nucleosides, applied in the field of mutagenic nucleoside analogs, can solve the problems of severe reduction of the efficacy of those drugs, and achieve the effects of reducing or avoiding symptoms, signs or causes of the condition, and reducing or minimizing one or more symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
IR Spectroscopy Tests of KP1212 in Aqueous Solutions
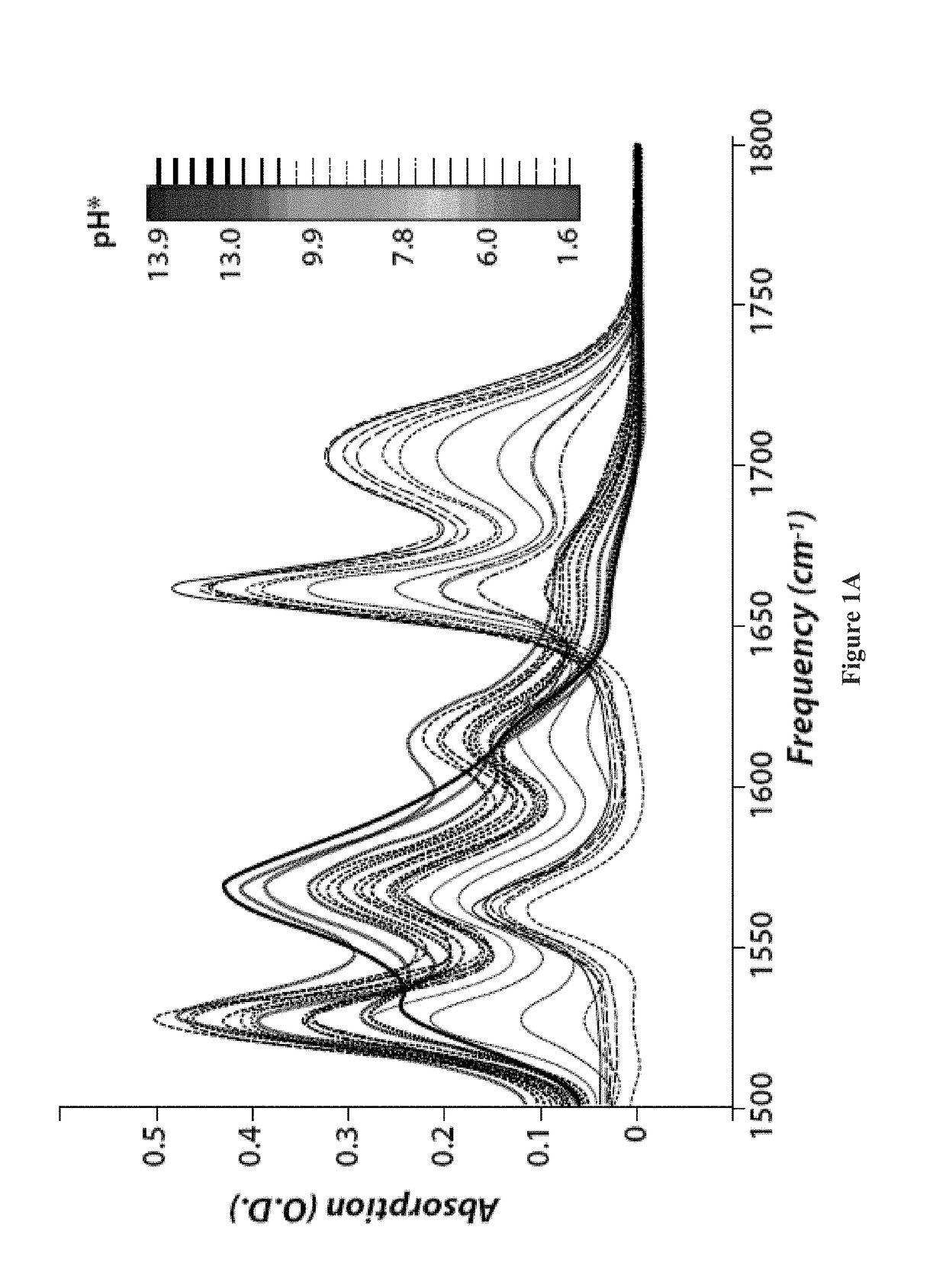
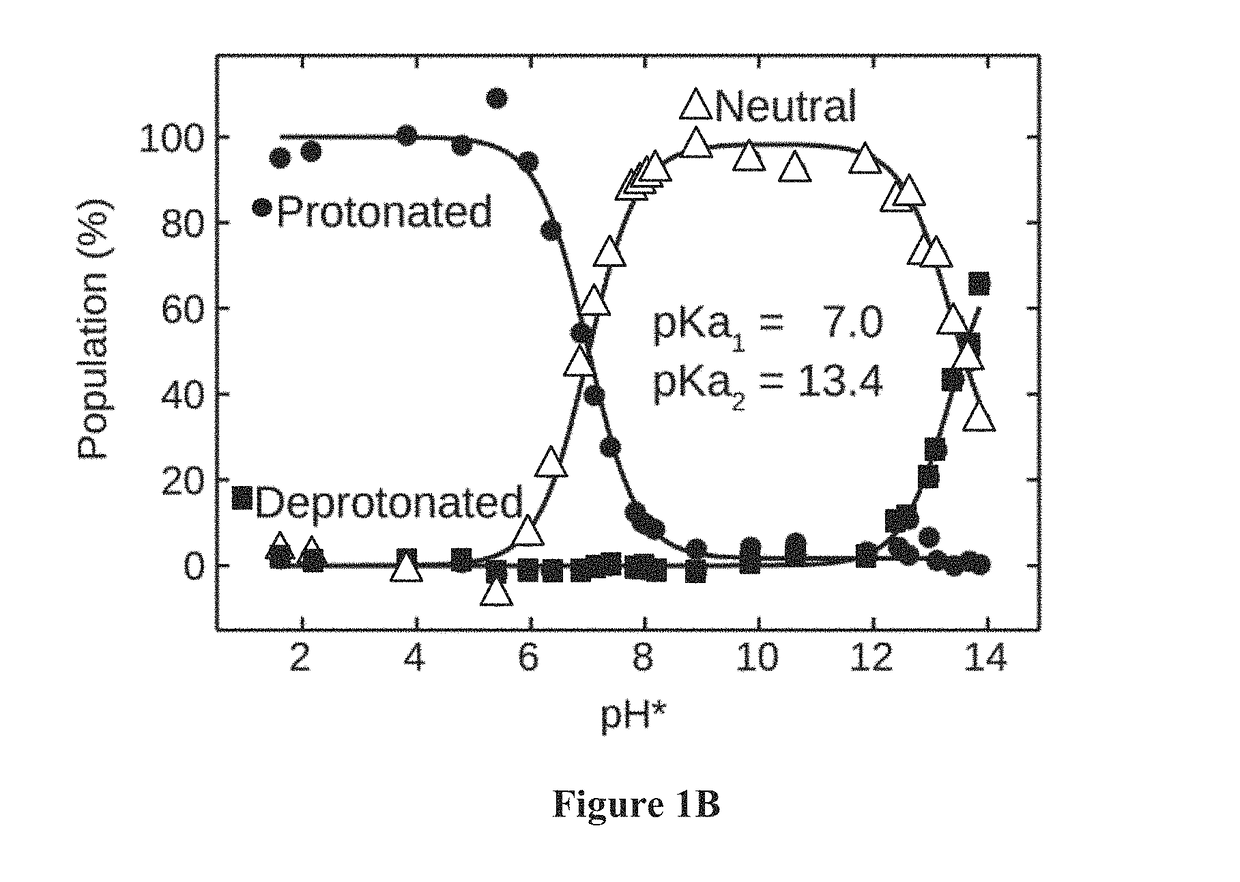
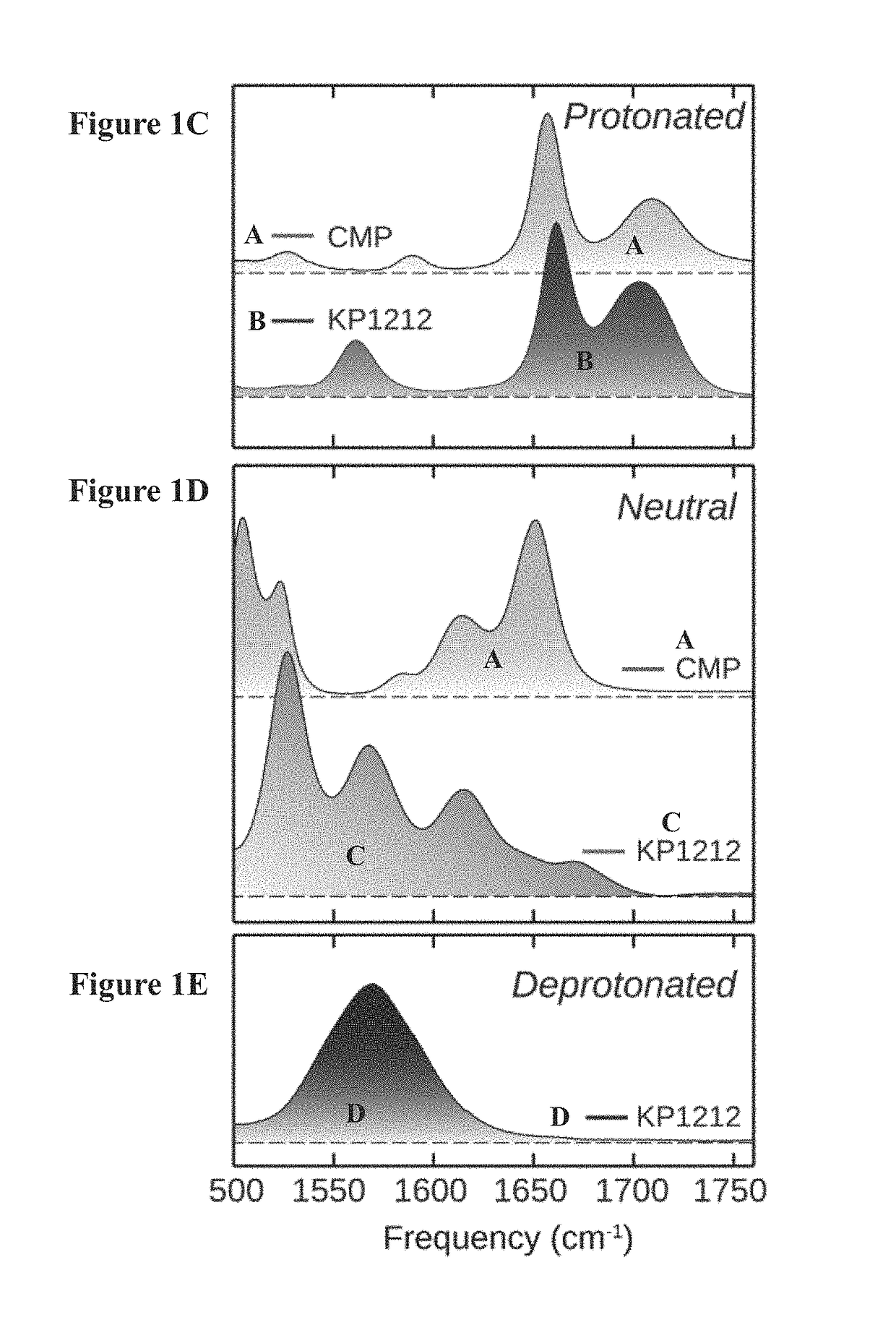
[0258]Methods. For both 1D FTIR and 2D IR experiments, the H / D exchanged KP1212 was dissolved at a concentration of 20 mg / ml (88 mM) in 0.5 M phosphate buffer pD (pH reading in D2O) 7.9. About 25 μl of sample solution was sandwiched between two CaF2 windows separated by a 50 μm TEFLON spacer. Variable-temperature FTIR spectra were collected using Nicolet 380 FTIR spectrometer at 1.0 cm−1 resolution with 16 scans per spectrum. The pH of the sample solution was varied (e.g., from 1.6 to 13.9, e.g., at 1.6 and 7.4, at 25° C.). Spectra for both the sample and the D2O were collected with the same procedure and the solvent spectra were subtracted from the sample spectra.
[0259]Absorptive 2D IR spectra were collected using a 2D IR spectrometer as described in detail previously (Chung, H. S., Khalil, M., Smith, A. W. & Tokmakoff, A. Transient two-dimensional IR spectrometer for probing nanosecond temperature-jump kinetics. Rev. Sci. Instrum...
example 2
Mutagenesis of KP1212 Depends on pH
[0262]The M13 single-stranded genomes, containing one KP1212 base at a specific site were constructed and purified as previously reported (Li, Fedeles, Singh, Peng et al, 2014, Tautomerism provides a molecular explanation for the mutagenic properties of the anti-HIV nucleoside 5-aza-5,6-dihydro-2′-deoxycytidine, Proc. Natl. Acad. Sci. U.S.A. 111, E3252-3259).
[0263]Primer extension reactions at various pHs were carried out as follows: 100 fmol extension primer (5′-CGTGATCATGCGCAGACTGACATCATGTGTAAAACGACGGCC AGTGAATTGGA-3′) were annealed to 100 fmol M13 genome by heating the mixture at 80° C. for 5 min followed by a slow (0.1° C. / s) cooling to 4° C. The primer was extended by 2.5 U of a suitable polymerase (for example Klenow) in a solution containing 50 mM phosphate buffer (pH adjusted from 5.5-8.5 in 0.5 increments), 100 mM K+ (adjusted with KCl), 10 mM Mg2+, 1 mM DTT and 125 μM of each of the four dNTPs for 4 h at 30° C. The resulting product was p...
example 3
Binding Isotope Effect (BIE) for Determining the Tautomeric Forms of a Ligand (OxyTPP) Bound to its Target Macromolecule (TPP Riboswitch)
[0266]Experimental method for using BIEs to determine tautomeric form of OxyTPP bound to the TPP riboswitch. The spectroscopic approaches described above allowed the full characterization of the tautomeric forms of nucleic acid base, nucleoside, nucleotide, and their analogs in the unbound form. Given the complexity of the nucleic acid polymers aptamer, these approaches could not be directly applied to determine the tautomeric form of OxyTPP bound to the riboswitch. To establish the tautomeric form of the bound OxyTPP, experimental binding isotope effects (BIEs) were used, which characterize the increase or decrease in binding upon substitution of an atom with its heavier isotope. BIEs are useful as they are sensitive to change in bond order between two equilibrium states and are influenced by alterations in vibrational frequencies between the boun...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com