Biological adhesive lung aspiration nanometer composite microparticle and preparation method thereof
A technology of bioadhesion and composite particles, which is applied in the field of medicine, can solve the problems of inconsistent drug dissolution rate, difficult mixing process, low preparation deposition rate, etc., and achieve the effects of anti-scavenging, prolonging the residence time of the lungs, and easy dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
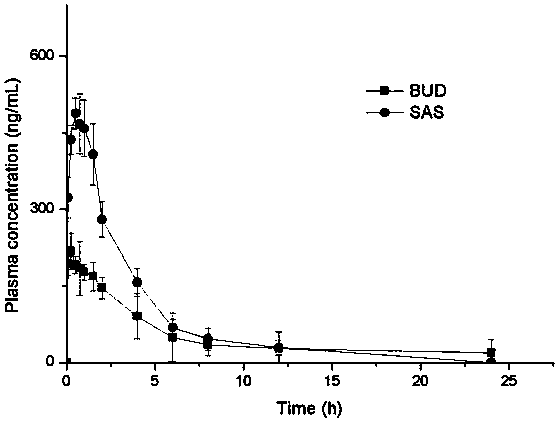
[0058] Example 1: Preparation of bioadhesive compound lung inhalation nanocomposite particles (SAS-BUD-HA / MS) comprising salbutamol sulfate (salbutamol sulfate, SAS) and budesonide (budesodine, BUD) nanocrystals
[0059] Weigh 0.5 g of hyaluronic acid, add 100 mL of double-distilled water, stir magnetically until fully swollen and dissolved, then add 250 mg of albuterol and 2 mL of a suspension containing 100 mg of budesonide nanocrystals with a particle size of 200 ± 2 nm, and continue stirring to make the drug Disperse evenly and spray dry. As a control, the raw materials of salbutamol sulfate and budesonide were added in proportion to redistilled water (8%, w / v), dispersed evenly, and spray-dried. The conditions of spray drying are: inlet temperature 160°C, dry air flow rate 35m 3 / h, atomization pressure 414L / h, liquid feed rate 4.5mL / min, prepared bioadhesive pulmonary inhalation nanocomposite particles containing albuterol and budesonide nanocrystals.
[0060] The aero...
Embodiment 2
[0063] Example 2: Preparation of bioadhesive lung inhalation nanocomposite particles (ST-FP-HA / MS) comprising salmeterol (Salmeterol, ST) and fluticasone propionate (Fluticasonepropionate, FP) nanocrystals
[0064] Weigh 1.5g of hyaluronic acid, add 150mL double-distilled water, stir magnetically to make it fully swell and dissolve, then accurately add 120mg of salmeterol and 600mg of fluticasone propionate nanocrystalline suspension with an average particle size of 200±10nm Solution 50mL, continue to stir, make two kinds of medicines disperse evenly in the solution, carry out spray drying. As a control, salmeterol and fluticasone propionate raw materials were added in proportion to redistilled water (10%, w / v), dispersed evenly, and spray-dried. The spray drying conditions are all: the spray drying conditions are all, the inlet temperature is 120°C, and the drying air flow rate is 35m 3 / h, atomization pressure 414L / h, liquid inlet speed 6.0mL / min, obtain the bioadhesive lun...
Embodiment 3
[0068] Example 3: Preparation of bioadhesive lung inhalation nanocomposite particles (VT-FF-CS / MS) comprising Vilanterol (VT) and fluticasone furoate (Fluticasonefuroate, FF) nanocrystals
[0069] Weigh 1.0 g of chitosan as a pharmaceutical excipient, add 150 mL of 0.5% acetic acid aqueous solution and stir until dissolved, then accurately add 67 mg of vilanterol and 50 mL of fluticasone furoate nanocrystal suspension containing 533 mg, stir evenly, and spray dry. As a control, the raw materials of vilanterol and fluticasone furoate were dispersed in double distilled water (5%, w / v) at a ratio of 1:8 for spray drying. Spray drying conditions are: inlet temperature 120 ℃, dry air flow 35m 3 / h, the atomization pressure was 357L / h, and the liquid feed rate was 4.5mL / min, to obtain bioadhesive pulmonary inhalation nanocomposite particles containing itraconazole nanocrystals.
[0070] The aerodynamic particle size and the fraction of fine particles (FPF value) with particle size ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com