Solid electrolyte separator bonding agent
a solid electrolyte separator and bonding agent technology, applied in the manufacture of secondary cells, cell components, cell component details, etc., can solve the problems of chemical incompatibility with lithium metal negative electrodes, liquid electrolytes suffer, outgassing at high voltage, etc., and achieve the effect of lowering the interfacial impedan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Electrochemical Stack Having a Bonding Layer
[0248]In this example, a free standing gel electrolyte film was first prepared.
[0249]A blend of ethylene carbonate (EC) and propylene carbonate (PC) solvents was prepared in a 1:1 w / w ratio. The lithium salt lithium hexafluorophosphate was added to this mixture to achieve a 1M solution. To form the gel solution, 0.8 grams of a PVDF-HFP polymer (Kynar 2801) was mixed 2.8 grams of the lithium hexafluorophosphate solution and 8.5 grams of a solvent, THF (Tetrahydrofuran). The solution was cast via doctor blade onto a glass substrate inside a glove box. The film was allowed to dry in the glove box for 4 hours. The dry film thickness was 45 μm. This dry film was used as the gel electrolyte 103 below.
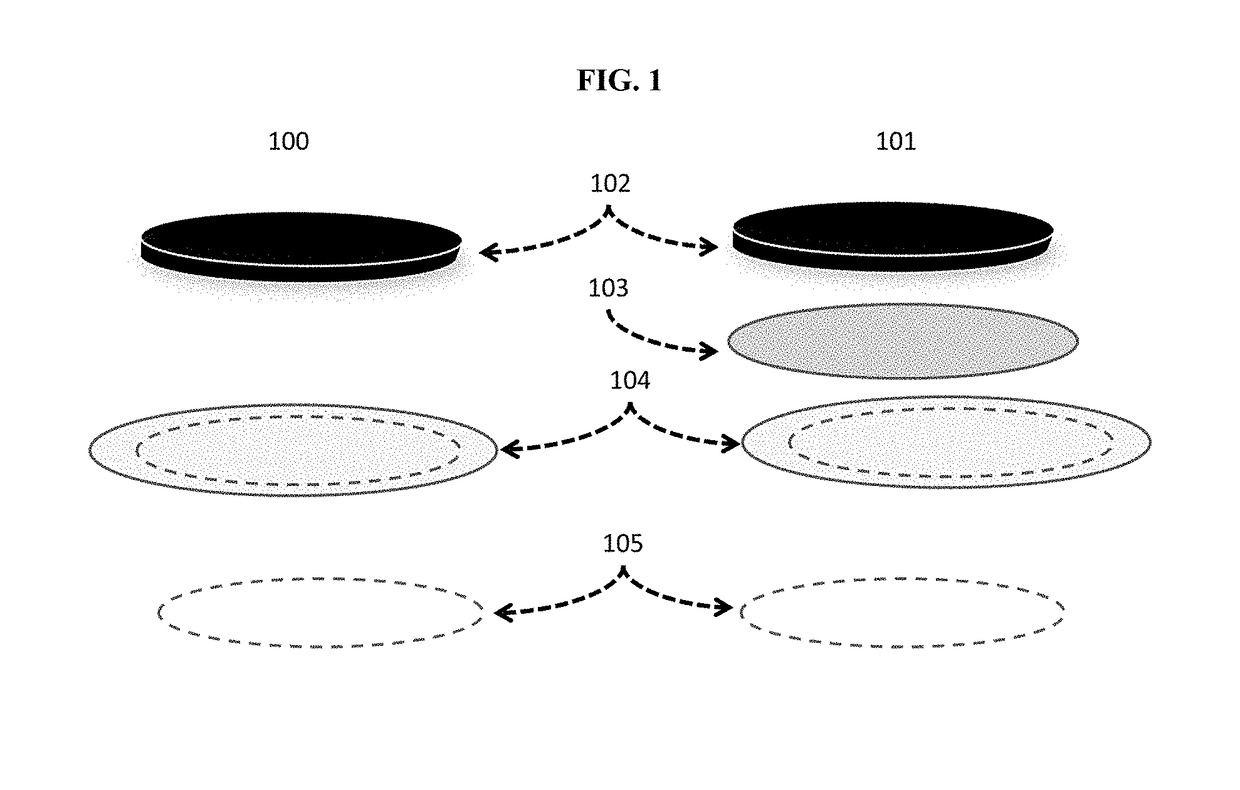
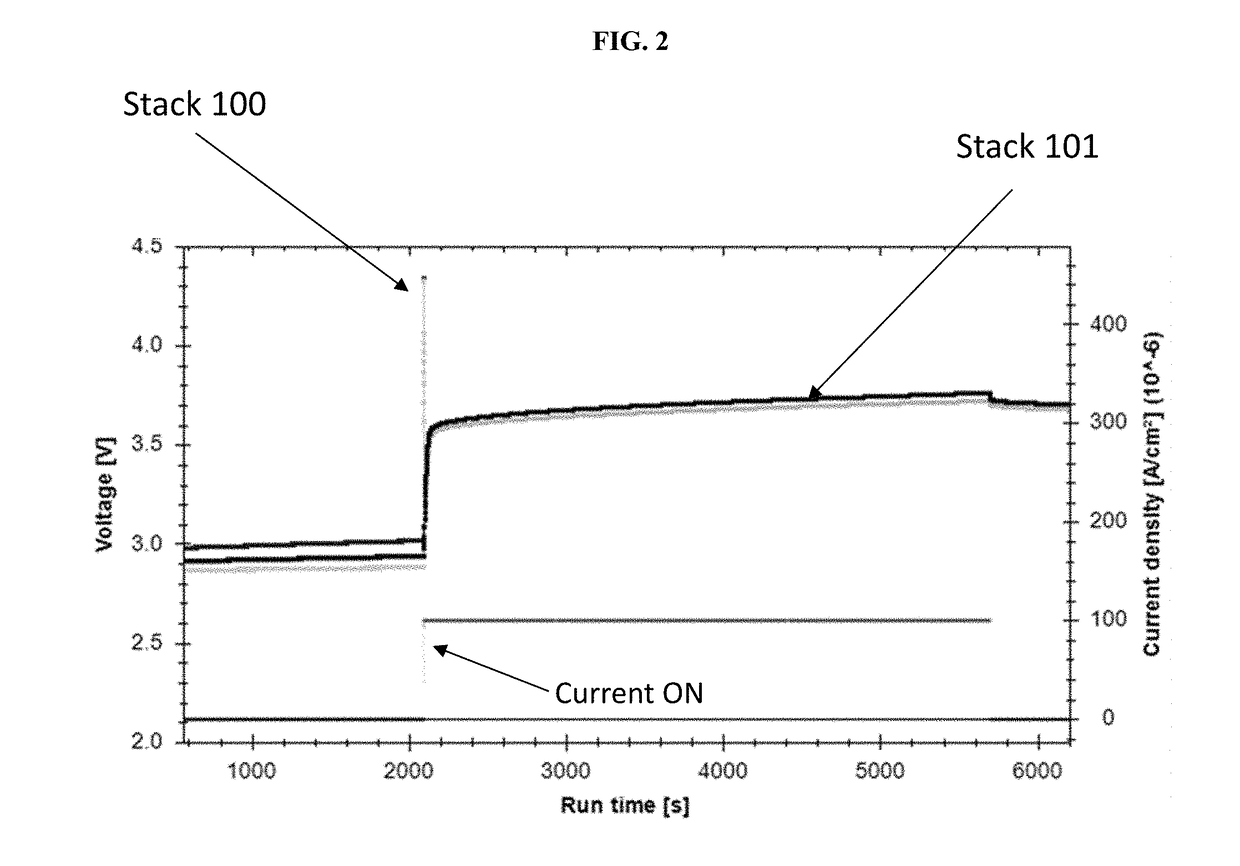
[0250]As shown in FIG. 1, two electrochemical stacks were prepared. The layers were pressed together in the actual electrochemical stack, but in FIG. 1 the layers are separated for illustrative purposes.
[0251]In one example, 100, the electrochemical...
example 3
Spin Coated Gel Bonding Layer
[0256]A blend of ethylene carbonate (EC) and propylene carbonate (PC) solvents was prepared in a 1:1 w / w ratio. The lithium salt, LiPF6, was added to this mixture to achieve a 1M solution. PAN polymer was mixed with the solution in a measured volume ratio PAN to EC:PC. The solution was spin-cast using a Laurel Technologies, Spincoater for up to 60 seconds to form a film. By varying the spin-cast RPM(s), thicker or thinner free standing films were prepared. The film was allowed to dry at room temperature on a garnet substrate for twenty-four hours. FIG. 4 shows the thickness of a PAN-containing film, which is as a function of the spin-cast RPM. The scale bar in FIG. 4 is 5 μm. Higher RPM results in thinner films. FIG. 5 shows a 47.4% PAN gel electrolyte which was spin-cast at 2000 RPM. The gel electrolyte, 501, is positioned on top of the garnet separator, 502. The scale bar in FIG. 5 is 100 μm.
example 4
ndividual Electrochemical Layers with Spin Coated Gel Bonding Layer
[0257]Determination of the interfacial resistance between a bonding layer and a garnet electrolyte requires measurement of a full cell resistance and subtraction of all other resistance components. The following experiments are used to determine the resistance of each layer and interface in a full cell so as to enable calculation of the interfacial resistance between a gel and a garnet separator (ASRB-G). As shown in FIG. 6, an electrochemical stack was provided having Li metal 603, a solid lithium-stuffed garnet, 602, Li7La3Zr2O12Al2O3, 50 μm thick film, and a lithium metal electrode, 601. This configuration is referred to a symmetric cell Li|garnet|Li cell.
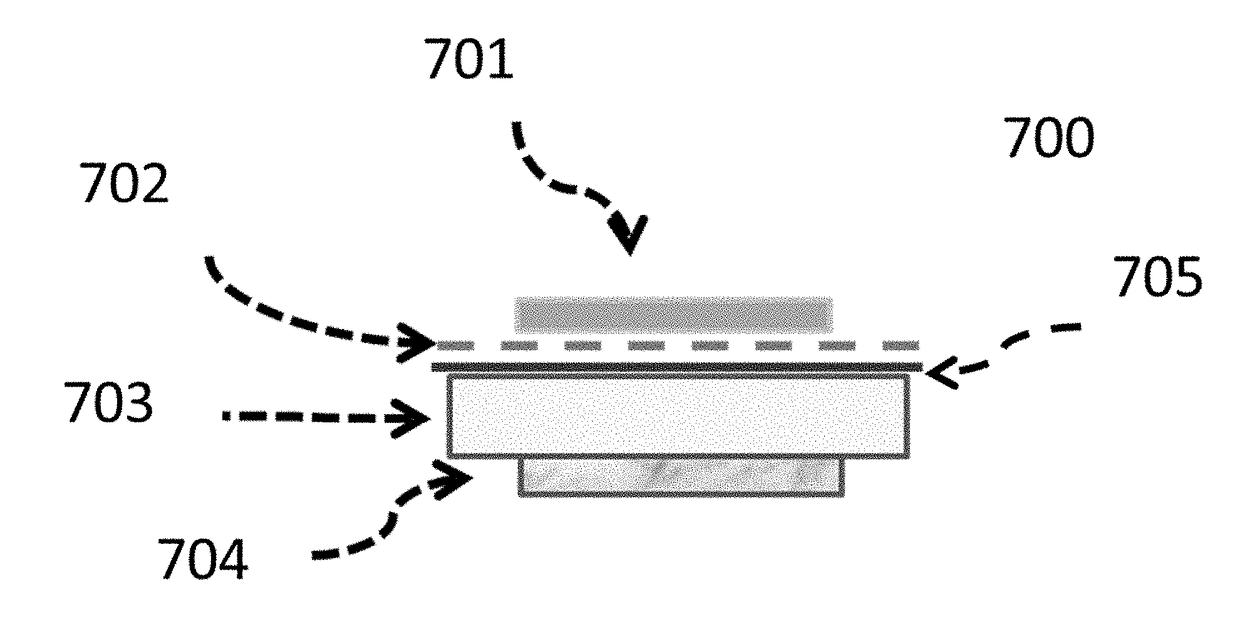
[0258]FIG. 7 shows a stack consisting of Li|Garnet|scGel|fsGel|Li-foil which was constructed and measured. Here, scGel refers to the spin-coat prepared gel electrolyte layer, 705 and fsGel refers to the doctor-blade coated free standing gel electrolyte layer, 702...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com