Diaryl carbonate and method for producing the same, and method for producing an aromatic polycarbonate resin
a technology of diaryl carbonate and aromatic polycarbonate, which is applied in the direction of carbonic/haloformic acid esters purification/separation, separation processes, organic chemistry, etc., can solve the problems of large amount of alkali, apparatus used in the production to suffer corrosion, and the time of polymerization can be reduced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0104]Hereinbelow, the present invention will be described in more detail with reference to the following Examples, which should not be construed as limiting the scope of the present invention.
[0105](Measurement of a BOD Concentration)
[0106]Each of the samples (BOD-containing DPC) obtained in Examples 1 to 10 was dissolved in acetone, and heptylbenzene as an internal standard material was added to the resultant solution, and subjected to quantitative determination by means of a gas chromatography analysis apparatus under the below-shown conditions for measurement. Substantially the same procedure as mentioned above was repeated to prepare sample solutions having respective known concentrations, and a calibration curve was prepared using the prepared solutions, and used in an analysis for a BOD concentration in DPC.
[0107]Measuring apparatus: Shimadzu GC-2014
Detector: FID
[0108]Column: GL Sciences Inc. TC-17 (0.30 m×0.25 mm I.D.),
Column temperature: 70° C. (5 min)−12° C. / min−190° C. (5...
examples 7 to 30
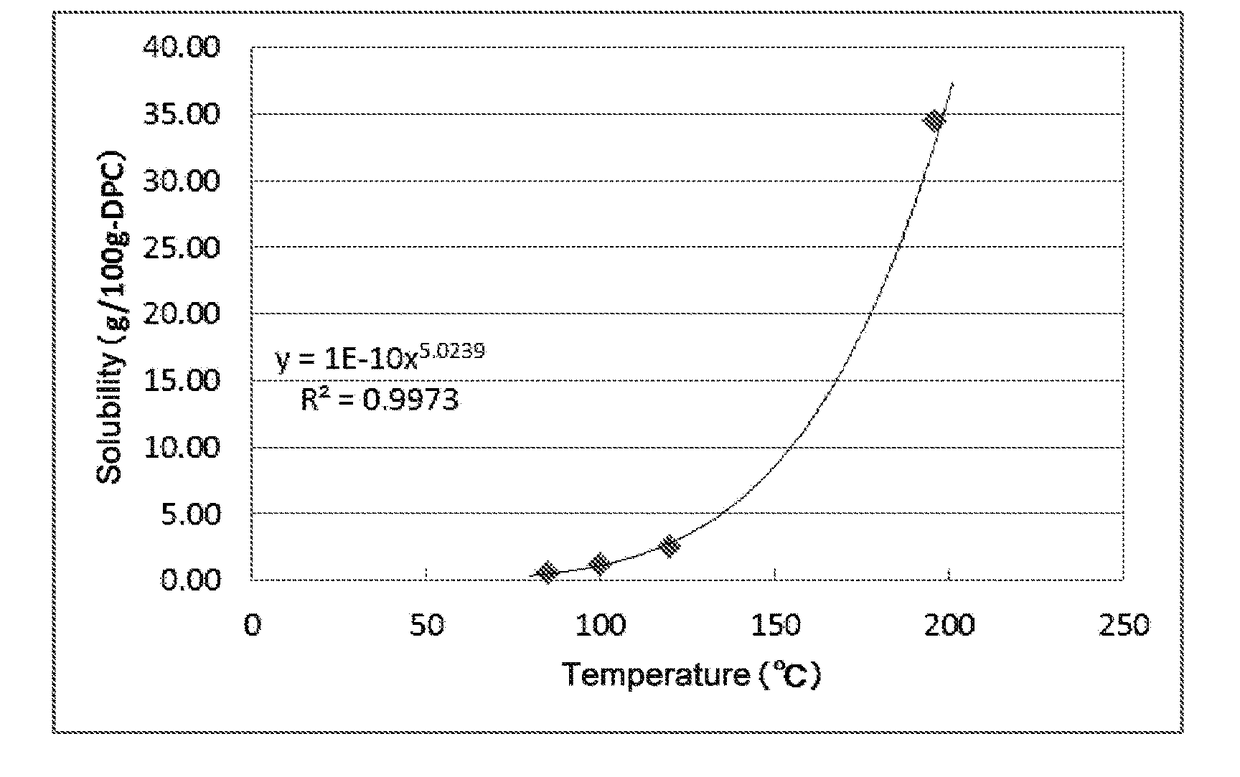
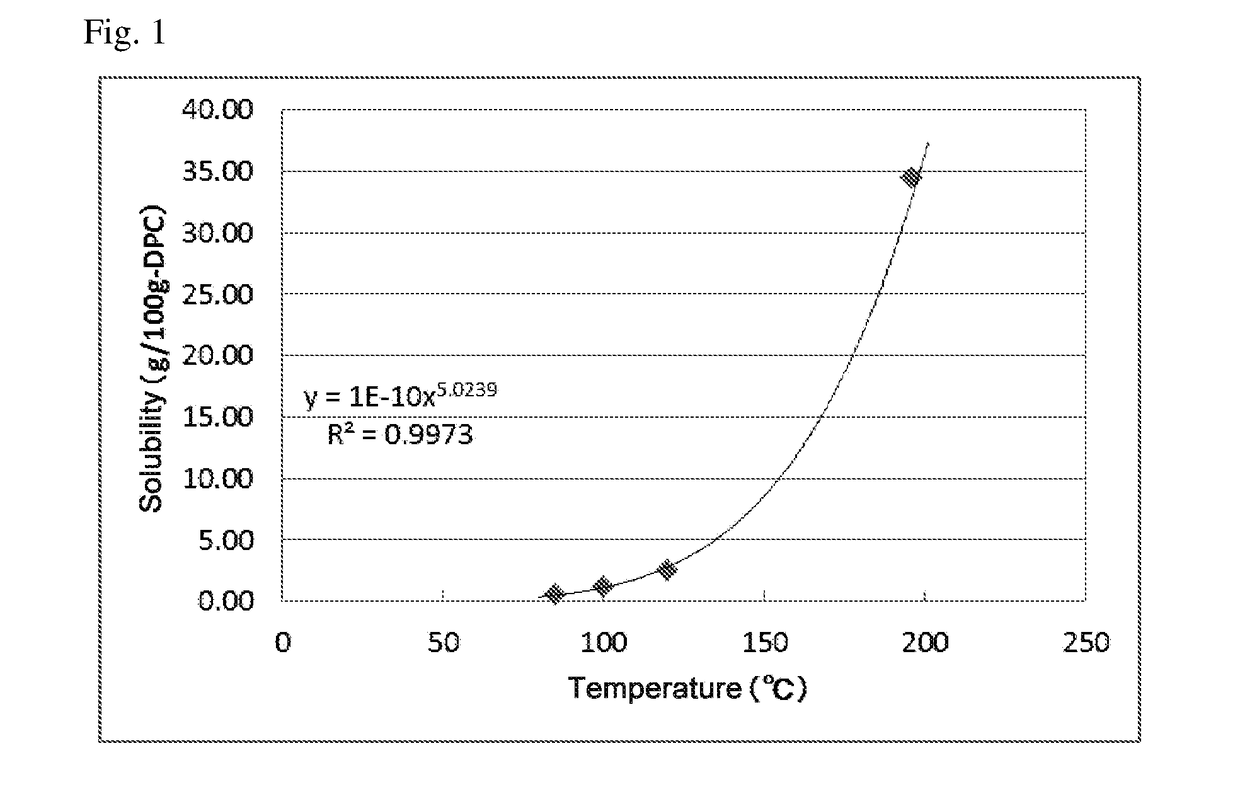
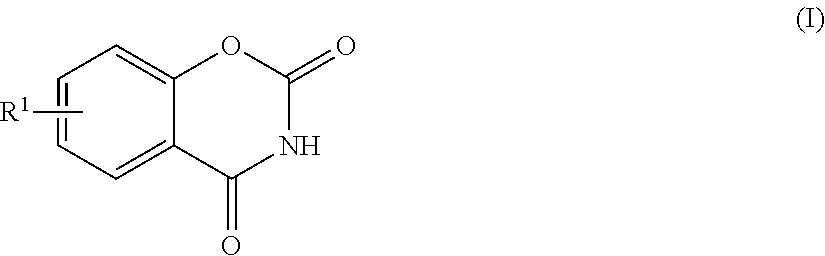
[0123]The distillation step in each Example was performed until the compound of the formula (I) in the liquid in the vessel was built up in such a concentration that the compound was precipitated when the temperature at the bottom of the column was reduced to 188.5° C. or lower. The resultant liquid in the vessel was withdrawn and then cooled so that the compound of the formula (I) was precipitated, followed by filtration under reduced pressure using No. 5C filter paper having a diameter of 330 mm, manufactured by Advantech Toyo Kaisha, Ltd., recovering a filtrate. The concentrations of the compound of the formula (I) and the precipitation conditions in the respective procedures are shown in Table 2. The solubility of BOD in DPC was measured and found to be as shown in FIG. 1.
TABLE 2Example789101112131415161718Example for distillation step123456123456BOD Concentra-GCwt %25.925.524.823.322.621.425.925.524.823.322.621.4tion in columnCompo-bottom DPCsitionPrecipitation° C.8282828282821...
example 31
[0124]The catalyst separation step and BPC recovery step in the purification step were performed in accordance with the same procedure as in Example 2. Then, the filtrate obtained in the precipitation step in Example 20 was added to the mixture obtained from the bottom of the BPC separation column in the BPC recovery step, and the resultant mixture was subjected to the distillation step in accordance with the same procedure as in Example 2. The BOD concentration (GC composition) of the obtained column top DPC was equivalent to the BOD concentration (GC composition) in Example 2 which includes no recycling.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com