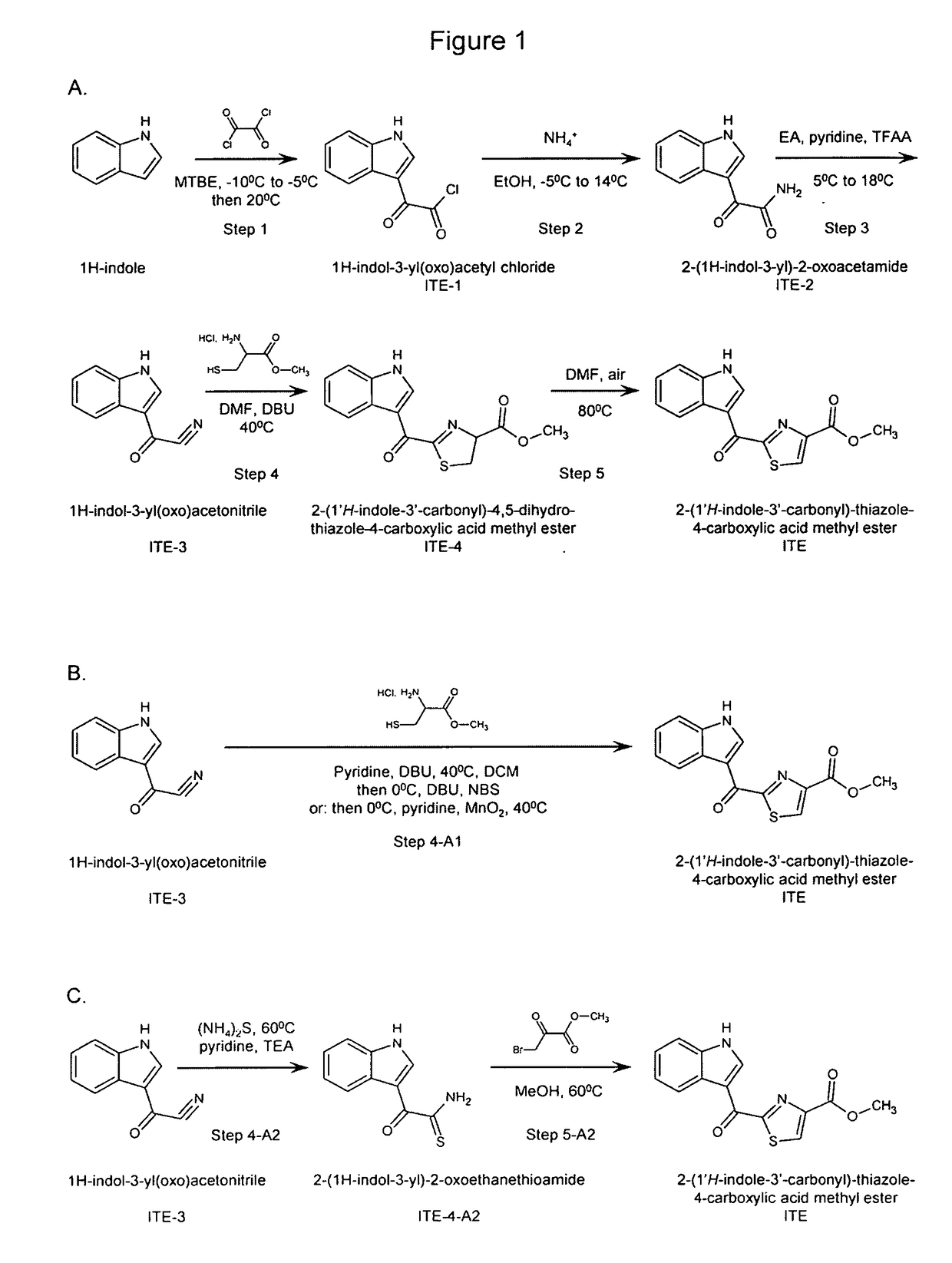
Efficient and scalable synthesis of 2-(1'H-Indole-3'-Carbonyl)-thiazole-4-carboxylic acid methyl ester and its structural analogs
a technology of thiazole and methyl ester, which is applied in the direction of organic active ingredients, cyano group formation/introduction, organic chemistry, etc., can solve the problems of inability to efficiently produce ite at levels, severe limits the efficiency of the entire synthesis, and unpredictable success of other cyclization reactions, etc., to achieve easy synthesis, increase the efficiency and scalability of synthesis, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0102]Example 1 shows a method of synthesizing ITE from 1H-indole via a number of intermediates, as depicted in Scheme A of FIG. 1.
Intermediate 1 (ITE-1): 1H-indol-3-yl(oxo)acetyl chloride
[0103]
[0104]1H-Indole (50 g, 0.43 mol.) and methyl tert-butyl ether (MTBE, 375 mL) were added to a three-necked round-bottom flask under stirring. The solution was cooled to −10° C., and then oxalyl chloride (56.9 g, 0.45 mol., 1.05 eq.) was added drop-wise while keeping the temperature between −10° C. and −5° C. The reaction mixture was then warmed to room temperature (−20° C.) and stirred at ˜20° C. for 1 hour. Petroleum ether (PE, 375 mL) was added to the reaction mixture. The suspension was stirred at ˜20° C. for 30 min. and then filtered. The filter cake was washed with PE (100 mL) and solvents in the cake were evaporated to give 108 g of product as a yellow solid. LC / MS: 208.6[M+1]
Intermediate 2 (ITE-2): 2-(1H-indol-3-yl)-2-oxoacetamide
[0105]
[0106]ITE-1 (108 g, 0.52 mol.) was added portion-wi...
example 2
[0114]Example 2 shows methods of synthesizing ITE from 1H-indol-3-yl(oxo)acetonitrile (ITE-3) in one container (“one-pot”) without purification of intermediate 2-(1′H-indole-3′-carbonyl)-4,5-dihydro-thiazole-4-carboxylic acid methyl ester (ITE-4), as depicted in Scheme B of FIG. 1. The process is presented in two sets of conditions.
example 2a
Product (ITE): 2-(1′H-indole-3′-carbonyl)-thiazole-4-carboxylic acid methyl ester
[0115]
[0116]ITE-3 (1 g, 5.88 mmol.), L-cysteine methyl ester hydrochloride (1.01 g, 5.88 mmol.), pyridine (5 mL) and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, 90 mg, 0.587 mmol.) were added to a three-necked round-bottom flask under stirring. After stirring at 40° C. for 2 hours, the reaction mixture was diluted with dichloromethane (DCM, 140 mL), then cooled to 0°. To the mixture was added DBU (1.79 g, 1.18 mmol.), followed by N-bromosuccinimide (NBS, 1.15 g, 6.46 mmol.) portion-wise. After stirring at 0° C. for 1 hour, the mixture was quenched with IN aqueous hydrochloric acid (100 mL) and extracted with DCM (20 mL) twice. The combined DCM layers were washed with IN aqueous hydrochloric acid (50 mL) and brine (50 mL), dried over anhydrous Na2SO4, and concentrated to give 1.71 g of crude product as a pale yellow solid (yield: 86.9%). Dimethyl sulfoxide (DMSO) and NaHCO3 were tested as solvent and base, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com