Pneumococcal conjugate vaccine formulations
a conjugate vaccine and pneumococcal technology, applied in the field of pneumococcal conjugate vaccine formulations, can solve the problems of limited serotype coverage of prevnar® in certain regions of the world, and the complications of these diseases can be significan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
on of DTFB Carrier Protein
Use of Multimodal Anion Exchange Chromatography for DTFB Preparation
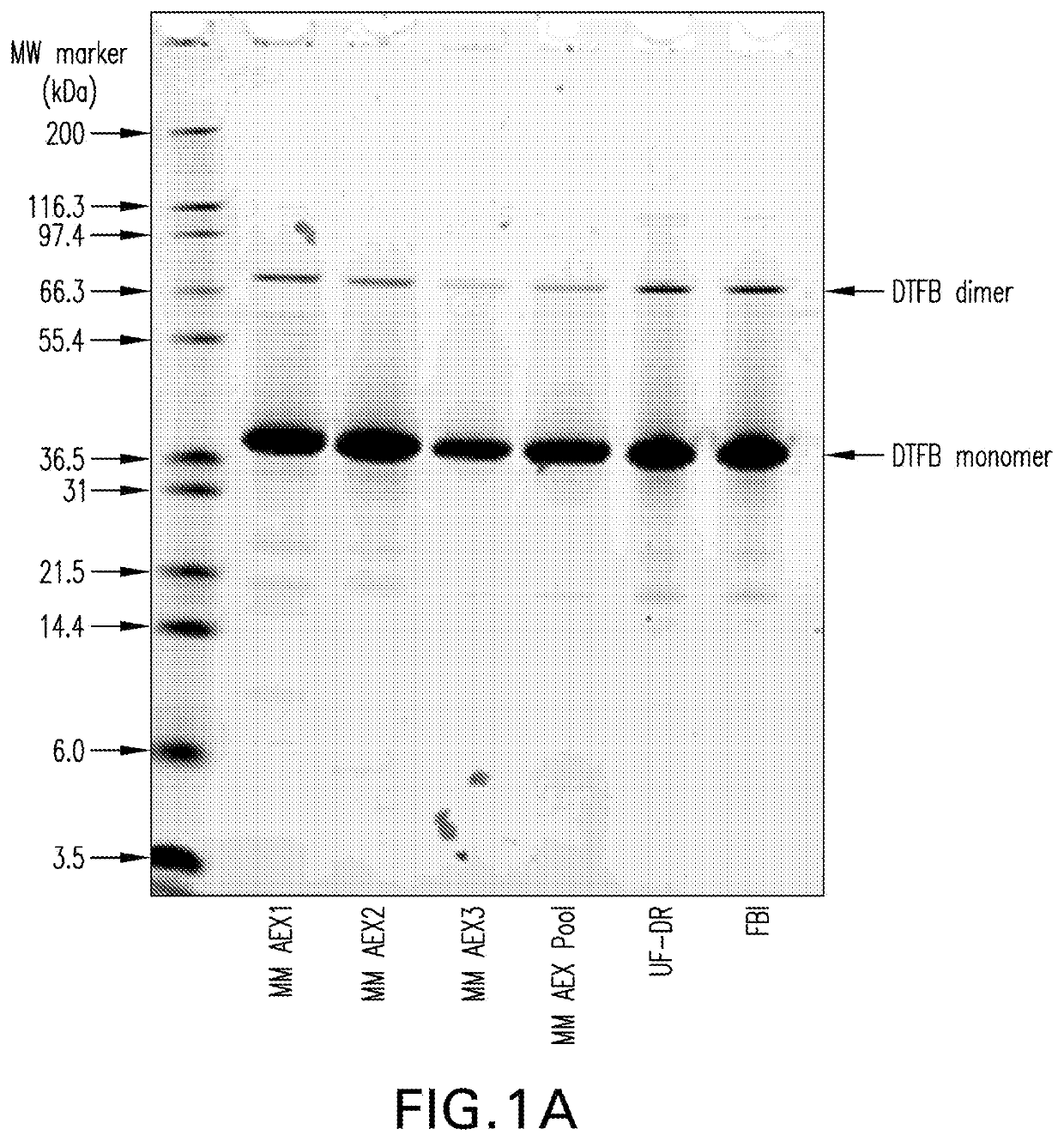
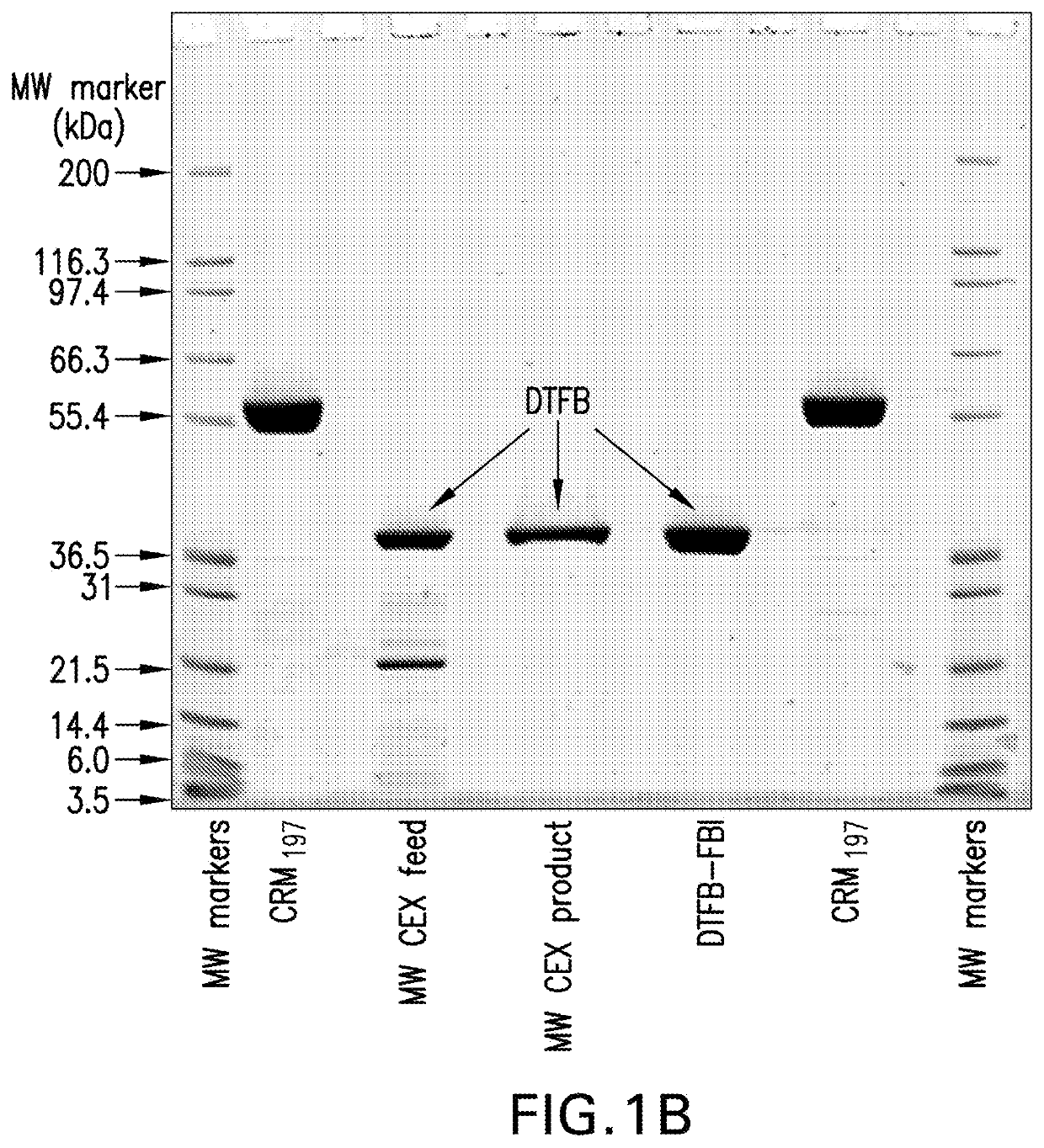
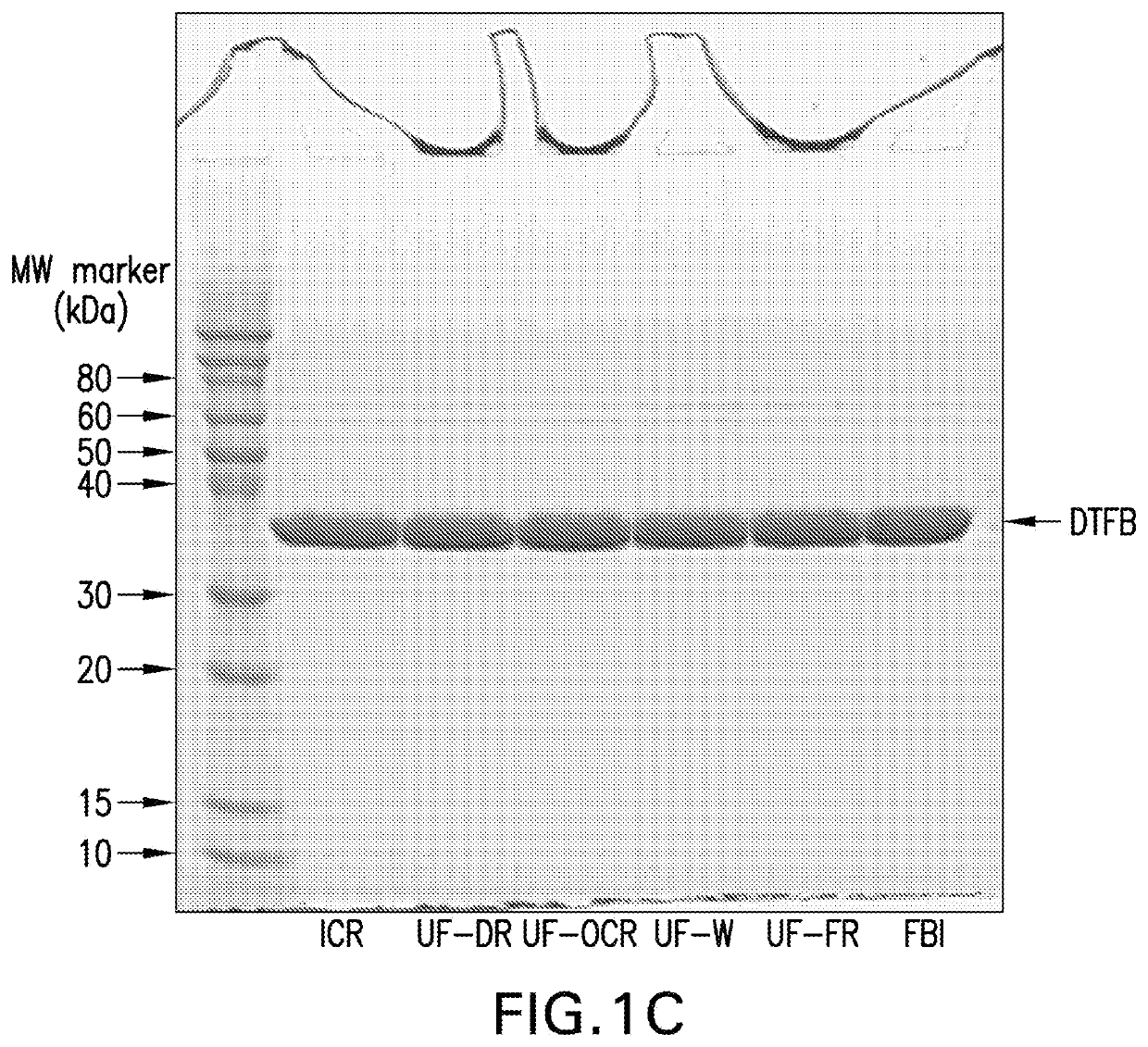
[0134]Purified CRM197, obtained through expression in Pseudomonas fluorescens as previously described (See International Patent Application Publication No. WO 2012 / 173876 A1), was digested with recombinant trypsin using a 1:500 molar ratio of trypsin to CRM197 for approximately 1 hour at approximately 22° C. in 50 mM Tris, pH 8.0. Dithiotheritol (DTT) in 50 mM Tris, pH 8 was then added to a final concentration of 5 mM for 30 minutes at approximately 22° C. to reduce the disulfide bond between the A and B fragments of the proteolytically-cleaved CRM197.
[0135]The digestion reaction was then loaded onto a multimodal anion exchange chromatography column (Capto™ Adhere, GE Healthcare) equilibrated with 50 mM Tris, pH 8. The column was washed with 50 mM Tris, pH 8, and the DTFB product was eluted with a gradient of 0.45 M to 0.65 M sodium chloride in 50 mM Tris, pH 8. The product was concentrated...
example 3
on of Polysaccharides to DTFB Carrier Protein Using Reductive Amination in Aqueous Solution
Preparation of Serotype 3-DTFB (ST3-DTFB) Conjugate for Mouse Immunogenicity Studies
[0151]Purified serotype 3 polysaccharide obtained as described in Example 2 was dissolved in water. Ps size reduction to an average molecular weight of approximately 200 kDa was performed using a probe sonicator with the sample cooled in ice. The sonicated sample was 0.2 micron-filtered and stored at 2-8° C. The polysaccharide solution was concentrated by diafiltration against a 30 kDa NMWCO tangential flow filtration membrane.
[0152]Polysaccharide was prepared for conjugation using sodium metaperiodate oxidation (See Anderson et al., 1986, J. Immunol. 137:1181-1186; and U.S. Patent Application Publication No. US20110195086). A 100 mM sodium metaperiodate solution was added to the polysaccharide solution in 50 mM sodium acetate. The sample was mixed for 14-18 hours at 19-25° C. protected from light. Ethylene gly...
example 6
unogenicity Study Using ST3-DTFB Monovalent Conjugate Formulation
[0182]The immungenicity of ST3-DTFB compared to ST3-CRM197 was evaluated in a mouse model. Adjuvanted formulations for administration to mice were prepared by mixing 24 μL of sterile-filtered conjugate (1:10 in saline—0.1258 mg DTFB or CRM197-conjugated polysaccharide per mL) with 62 μL of APA, and 3.664 ml of sterile saline for a dose of 0.08 μg of polysaccharide and 5 μg of aluminum per 100 μL. The formulated vaccines were stored in individual borosilicate stoppered vials at 2-8° C. to support individual immunizations.
[0183]ST3-DTFB was evaluated in 6-8 week old female Balb / C mice (n=10 / group). Mice were immunized with ST3-DTFB / APA and two ST3-CRM197 / APA lots made using unique ST3-DTFB and ST3-CRM197 conjugate preparations prepared as described in Examples 3 and 4. The ST3 PnPs concentration was 0.08 μg per dose in a 0.1 ml volume, with 5 μg of APA, given intraperitoneally on days 0, 14 and 28. Sera were collected pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com