Electrolyzer for gaseous carbon dioxide
a carbon dioxide and gaseous technology, applied in the field of electrochemical devices, can solve the problems of limited use of conventional electrochemical reduction systems, inefficient conventional systems, poor stability, etc., and achieve the effect of eliminating or reducing undesired crossover of chemical products, improving the transport rate of chemical species, and reducing the number of undesired chemical product crossovers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
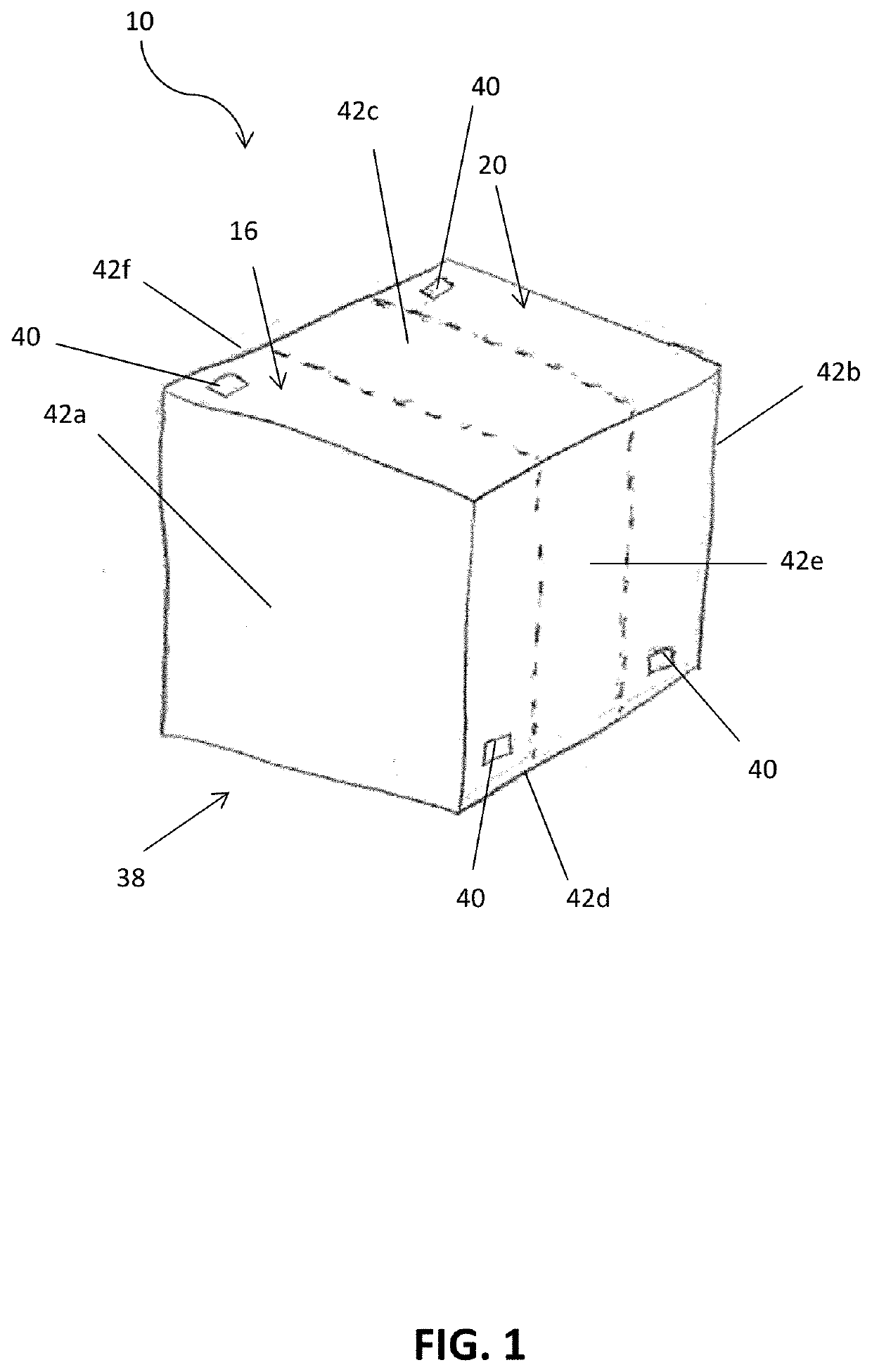
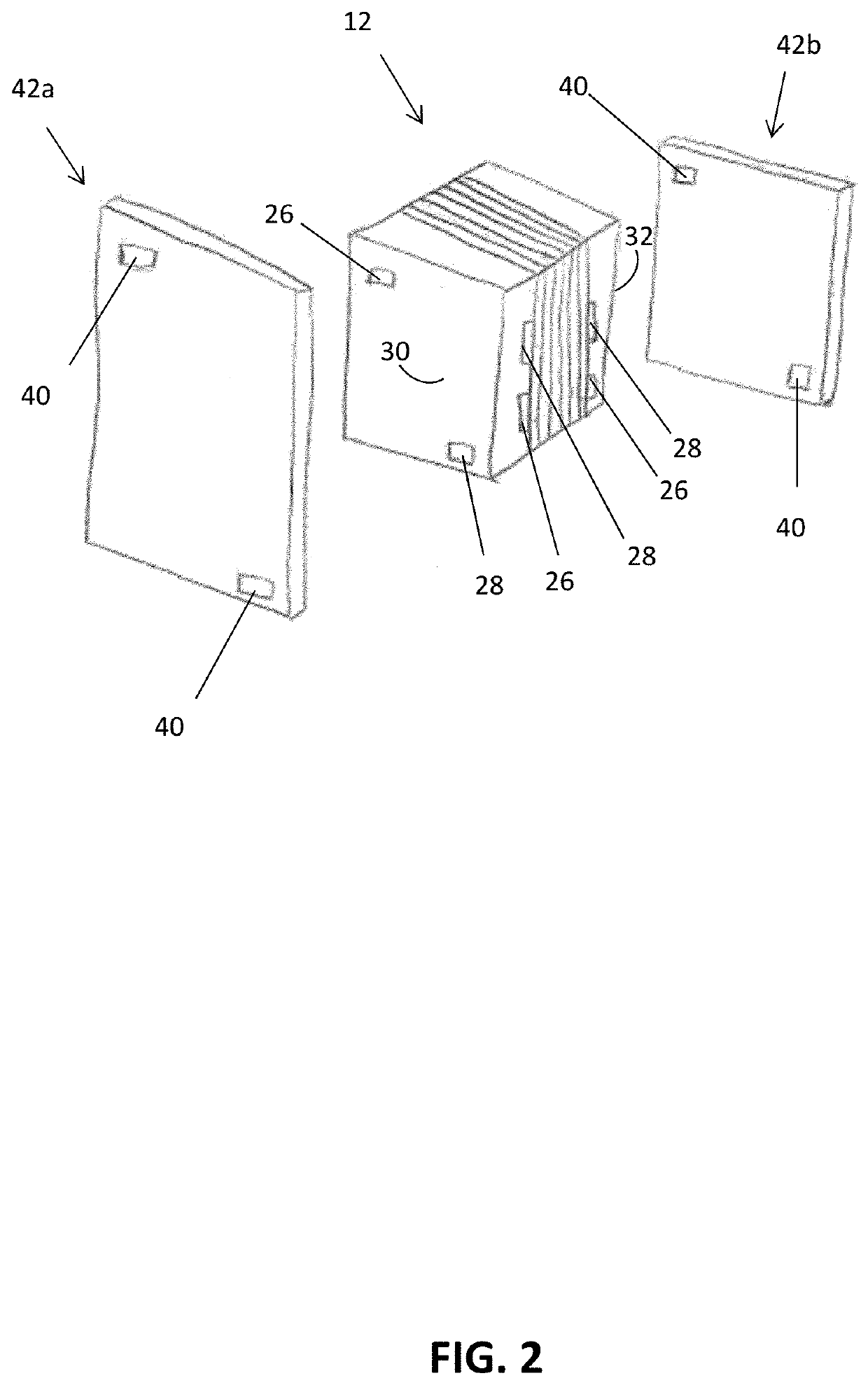
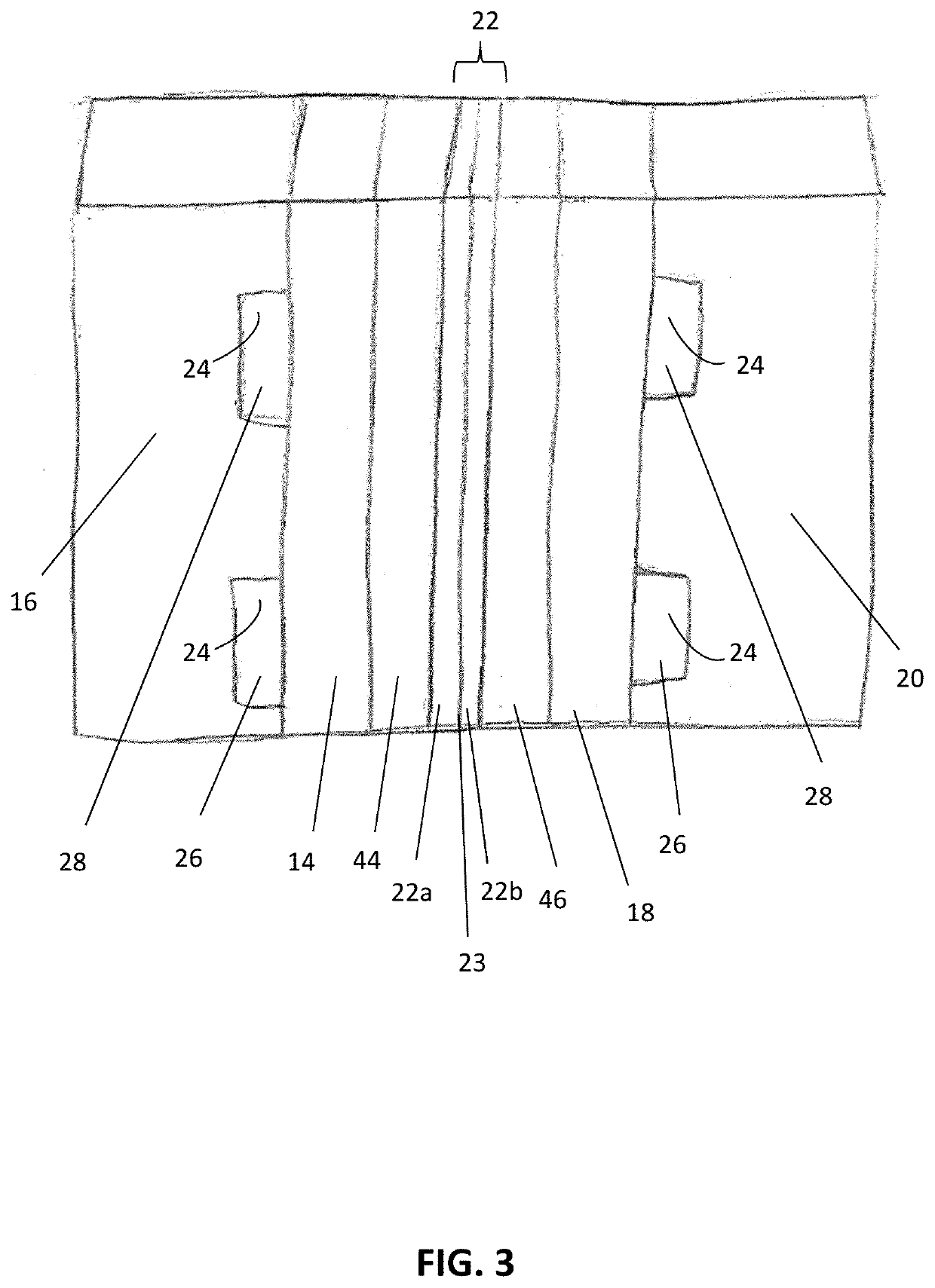
[0108]In a non-limiting example, an embodiment of the electrochemical device 10 was created using a gas diffusion cathode 14. The cathode 14 included a piece of Toray carbon paper (Toray® TGP-H-120). Silver nanoparticles (100 nm diameter, Sigma Aldrich®) were use as the CO2 reduction catalyst 44. Cathode catalyst ink 44 was made by mixing 8 miligrams of silver nanoparticles with 200 microliters of isopropyl alcohol, 200 microliters of deionized water (18.2 MΩ), and 15 microliters of 5% Nafion (sulfonated tetrafluoroethylene based fluoropolymer-copolymer solution), which was sonicated for 10 minutes. The cathode catalyst ink 44 was then painted onto the carbon paper at a typical loading of about 5 miligrams per square centimeter (mg / cm2). The anode 18 included a piece of Toray carbon paper (Toray® TGP-H-120). NiFeOx was used as the anode catalyst 46. NiFeOx was electrodeposited onto the carbon paper.
[0109]The anode 18 and cathode 14 were assembled together with a bipolar membrane 22....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com