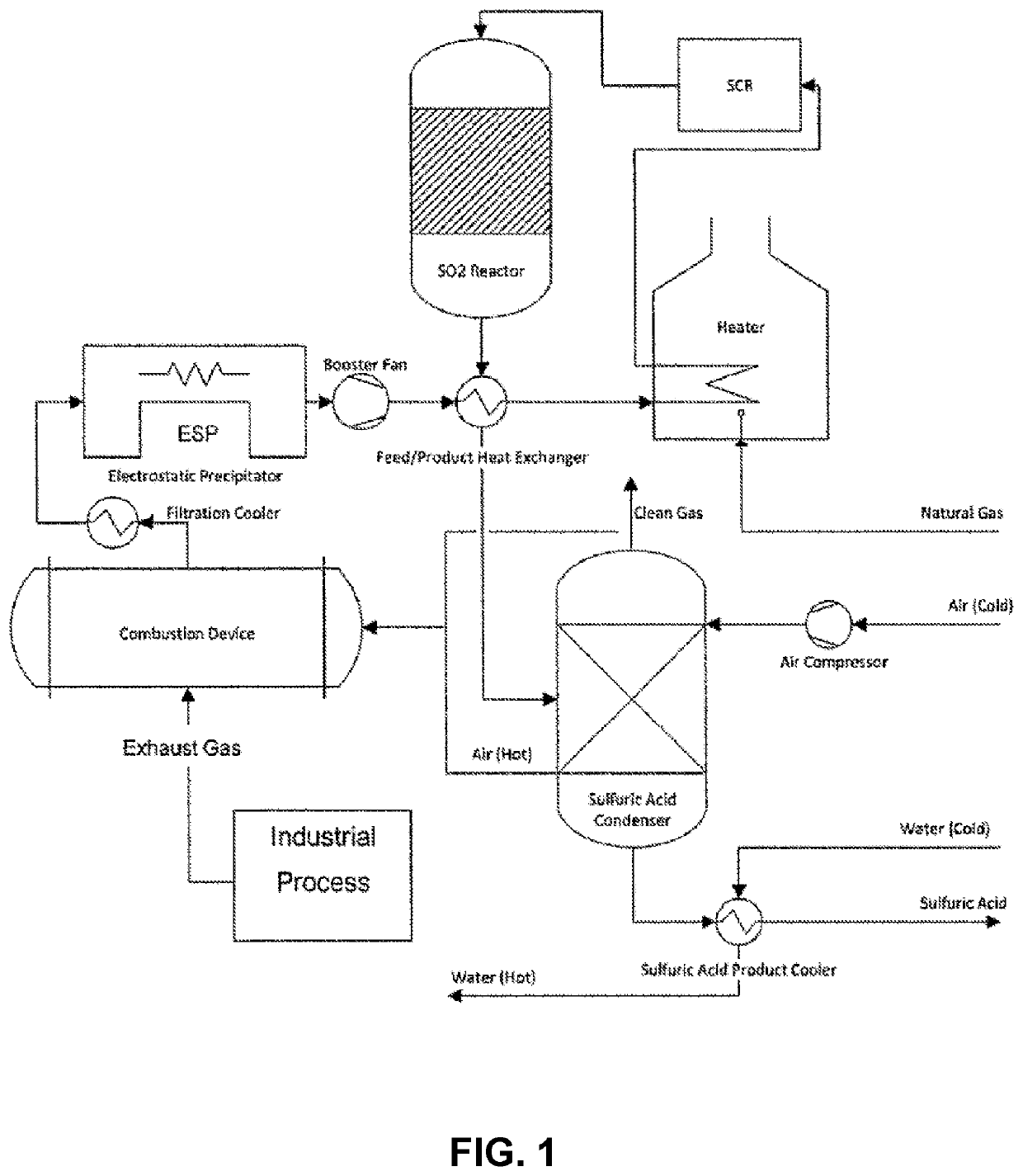
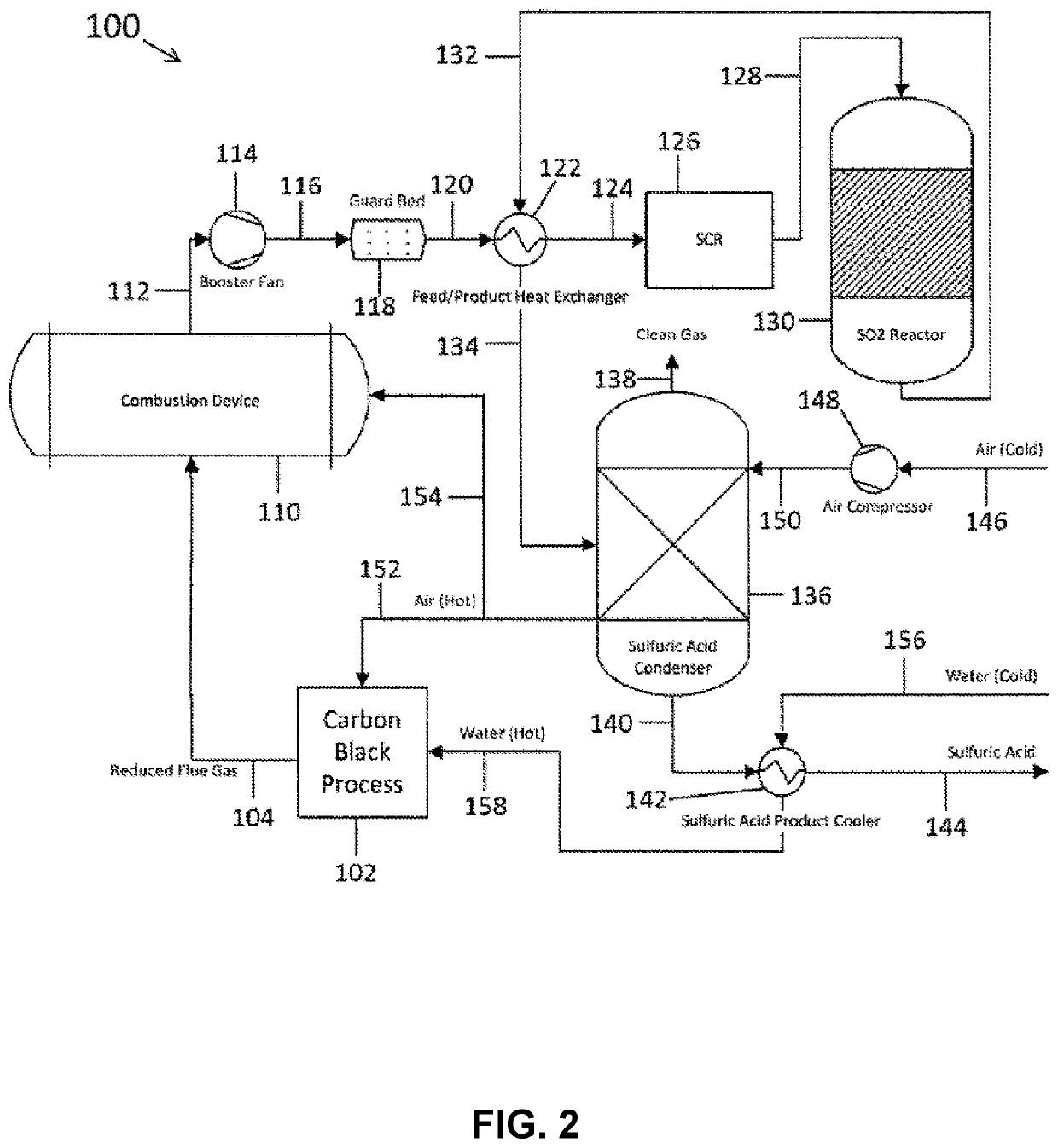
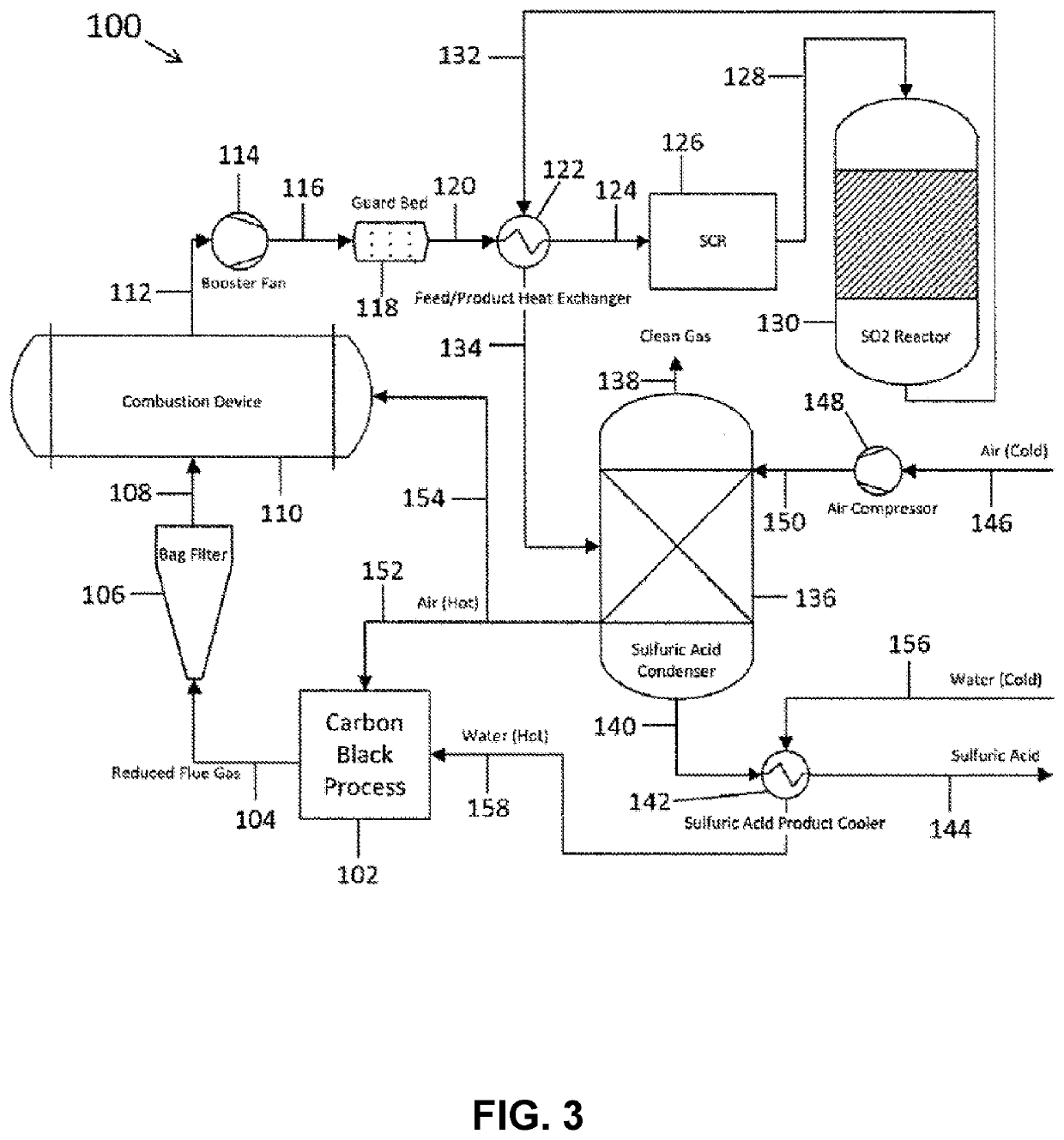
Methods and systems for particulate matter removal from a process exhaust gas stream
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0104]The first example evaluated whether there is pressure build up or poisoning of the catalyst when running at PM being multiple times higher than the 3 typically achieved after PM is removed from a combusted exhaust gas using electrostatic precipitation (ESP).
[0105]The first trial (1-A) was carried out with catalyst IV and a flow rate of 60 Nm3 / h, which is approximately a three times higher gas velocity inside the reactor than in commercial WSA plants and therefore intentionally overemphasizing possible negative effects of the PM. The operating conditions where:
[0106]SO2 50-500 ppmv, with an average of 225 ppmv
[0107]H2O>30 Vol %
[0108]O2 commonly around 1.2 Vol %
[0109]PMPM / kgcatalyst h
[0110]reactor temperature (gas phase) 420° C.
[0111]It was found that even after 28 days of operation neither the differential pressure of the guard bed (4.5 mbar), nor that over the reactor (12 mbar) increased. Subsequent kinetic testing of several samples across the length of the guard bed and acro...
example 2
[0119]These examples show the impact of the SO2 level on the conversion of the SO2.
[0120]The SO2 conversion at comparable process conditions (30 Nm3 / h flow, H2O>>30 Vol %, O2>>1 Vol %, 101 catalyst, Treaction=395° C., PM load PM / kgcatalyst h]) but varying SO2 levels is as in Table 2.
TABLE 2SO2 inletSO2 reactor outletSO2 conversionCatalyst[ppmv][ppmv][rel. %]I962475I1501590I310997I7352297I19505897II1607.395II12003897
[0121]These measurements validate that a common WSA catalyst is also active at process conditions typical for a CB plant. No test at higher SO2 loads (single digit percentage range) was carried out because the catalyst will be at least as active as at low SO2 levels, as long as the oxygen content is sufficiently high and the SO2 levels do not exceed typical WSA levels.
[0122]The experiments also indicate that at very low SO2 levels the choice of catalyst can impact the conversion significantly.
[0123]Examples 1 and 2 demonstrate that there is no (significant) difference in ...
example 3
[0124]Having shown that a guard bed can protect a conventional WSA process from poisoning by high levels of PM, the robustness of the process was demonstrated. The biggest threat for the guard bed / catalyst system would be an increase in the PM load, either by boiler operation or by a filter breach. To simulate these failure mode operations additional CB was spiked to the system, increasing the PM up to 500 mg / Nm3 for several hours. With a failure mode operation lasting only minutes this testing was intentionally overemphasizing the PM load, targeting to demonstrate the robustness of the system.
[0125]Trial 3-A was carried out with catalyst I, the SO2 inlet varying between 700-1100 ppmv and the gas flow kept constant at 30 Nm3 / h. Each step was applied for at least 3 h and a total of 278 g CB was added during the testing (Table 3).
TABLE 3PM loadPM spikingdPguard+reactorSO2 outlet[gPM / kgcatalysth][mg / Nm3][mbar][ppmv]013.9220.275014.2220.5310014.4260.7915016.7241.0620017.2261.3225016.625...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com