Use of akkermansia in the treatment of oral diseases
a technology of akkermansia and oral cavity, which is applied in the field of oral disease treatment, can solve problems such as tooth loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
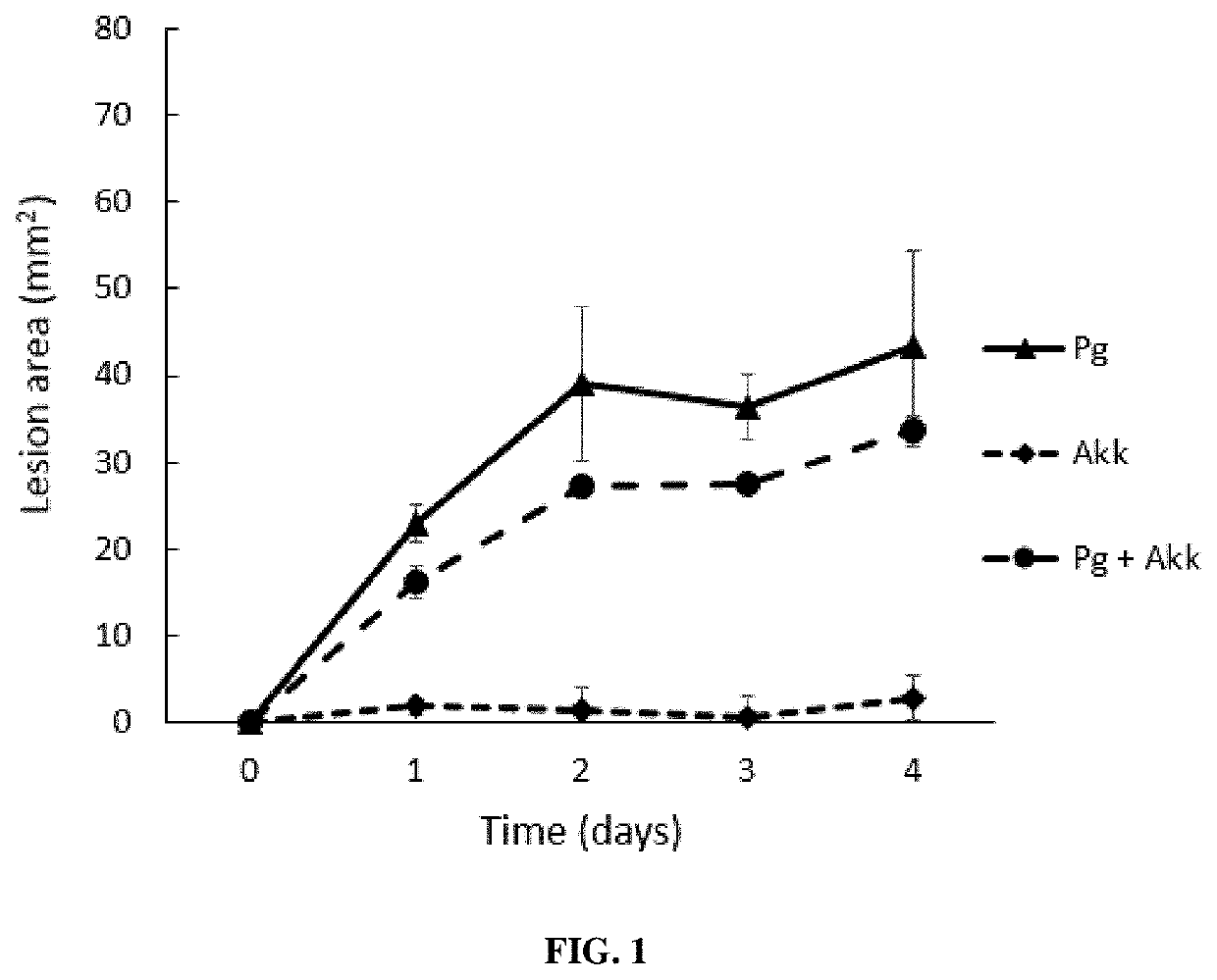
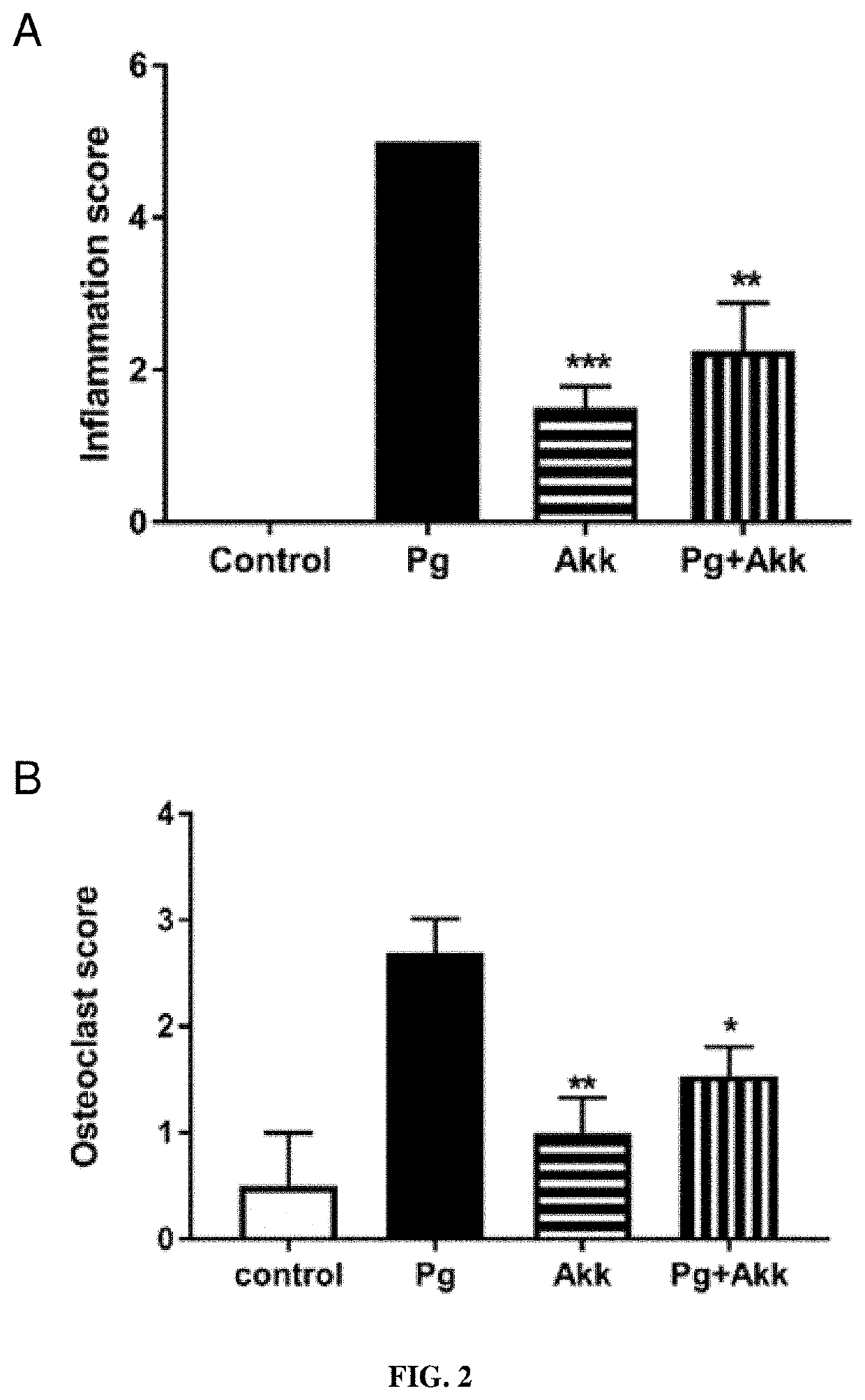
: A. muciniphila Decreases P. gingivalis-Induced Soft Tissue Inflammation and Calvarial Bone Destruction
Methods
Mouse Calvarial Bone Resorption Model
[0244]Mice were separated into the following four treatment groups (n=6): (i) PBS, (ii) P. gingivalis alone, (iii) A. muciniphila alone and (iv) P. gingivalis and A. muciniphila injected combined. Mice were anesthetized by the intraperitoneal injection of ketamine-xylazine (Akorn Animal Health, Lake Forest, Ill., USA). The heads of mice were shaved and cells of P. gingivalis (5×108), A. muciniphila (109) or no cells in 100 μL of PBS were injected subcutaneously in the 4 treatment groups with a 30.5-gauge needle at a point on the midline of the skull between the ears and eyes as described previously {Huck:2017gj}. Lesions were photographed each day for 5 days. Mice were sacrificed 5 days post injection. The size of the lesion resulting from the injection in each animal (area in square millimeters) was analyzed using ImageJ software.
Histol...
example 2
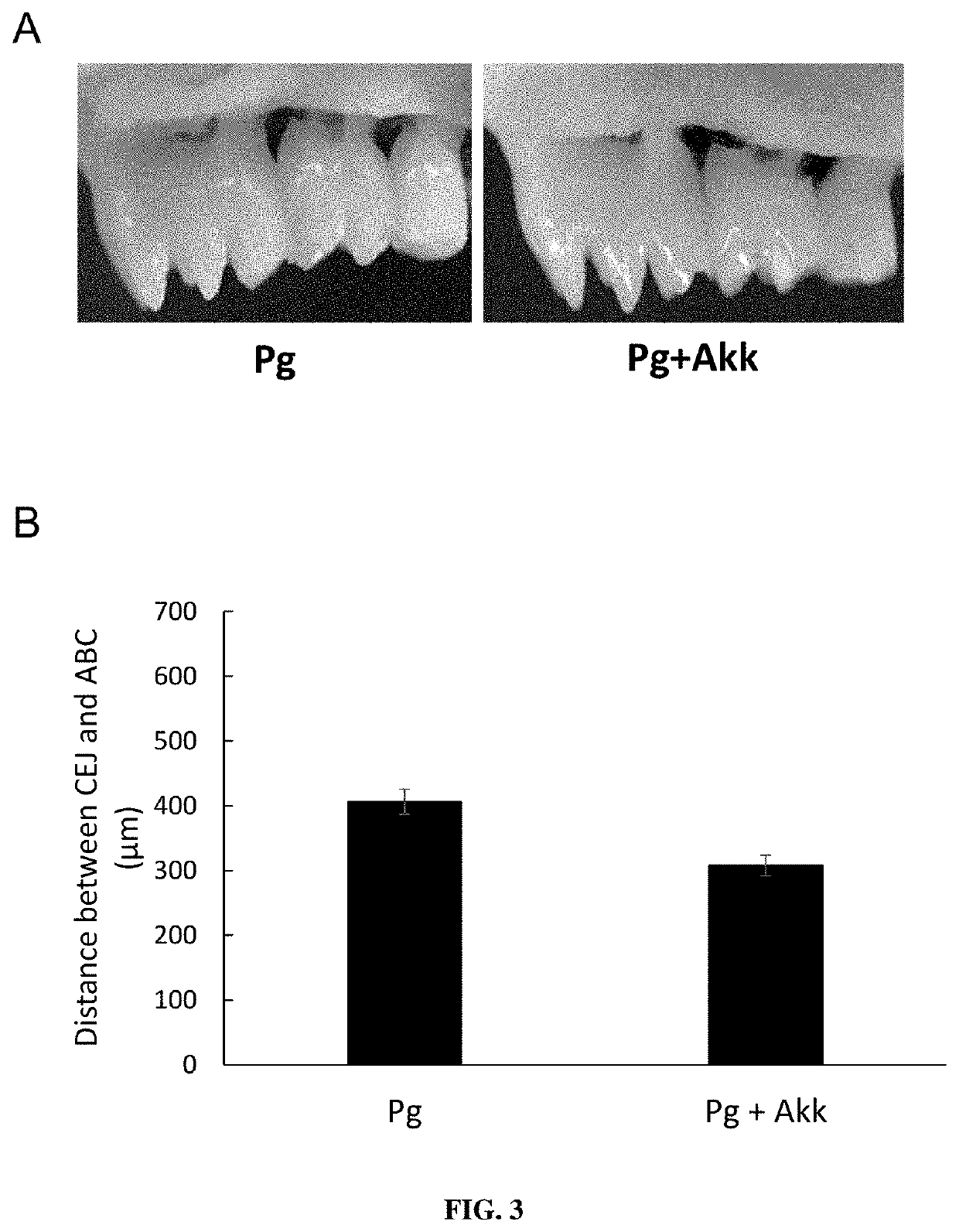
ic Administration of A. muciniphila Reduced P. gingivalis-Induced Alveolar Bone Destruction
Methods
[0263]Induction of Periodontitis and A. muciniphila Administration
[0264]Sterile black braided 6.0 silk sutures were incubated in bacterial culture medium with P. gingivalis for 1 day in anaerobic conditions. Following anesthesia, P. gingivalis soaked 6.0 ligatures were placed in the sulcus around the maxillary first and second molars as previously described {SaadiThiers:2012by}. Ligatures were replaced 3 times / week during 5 weeks. On days in-between ligature placement, oral gavage with P. gingivalis (5×108 cells) was performed as previously described {Alshammari:2017ip}. After 5 weeks of periodontitis induction, the ligatures were removed and mice were split in two groups (i) with daily oral gavage of P. gingivalis alone (5×108 cells) and (ii) with daily oral gavage of A. muciniphila (109 cells) and with P. gingivalis for an additional 2 weeks.
[0265]Palatal bone was...
example 3
: A. muciniphila Administration is Associated with a Shift of Periodontal and Gut Microbiota
Materials and Methods
Material
Oral and Fecal Sample Collection and Storage
[0269]Oral samples were collected by gently rubbing a sterile paper point over gums and teeth. Paper points were immediately placed into a sterile tube and stored at −80° C. Fresh fecal samples were collected from mouse into sterile tube and stored at −80° C. until analysis.
Methods
DNA Extraction, Sequencing and Data Processing
[0270]DNA was isolated from oral and fecal samples as described previously {Caporaso:2012fz}. Samples were placed in PowerBead Tubes and vortexed for 10 min at maximum speed using a 24-sample vortex adapter. The samples were processed following the manual and protocols according to the instructions in the DNeasy PowerSoil Kit Handbook (Qiagen, Calif.). The quality and quantity of DNA was determined by fluorometric quantification and by measuring A260 / A280 ratios. In addition, the DNA was visualized ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com