Parapoxvirus vectors
a parapoxvirus and vector technology, applied in the field ofviral immunotherapy, can solve the problems of a major obstacle to effective immunotherapy, and achieve the effect of improving the cytokine/chemokine profil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
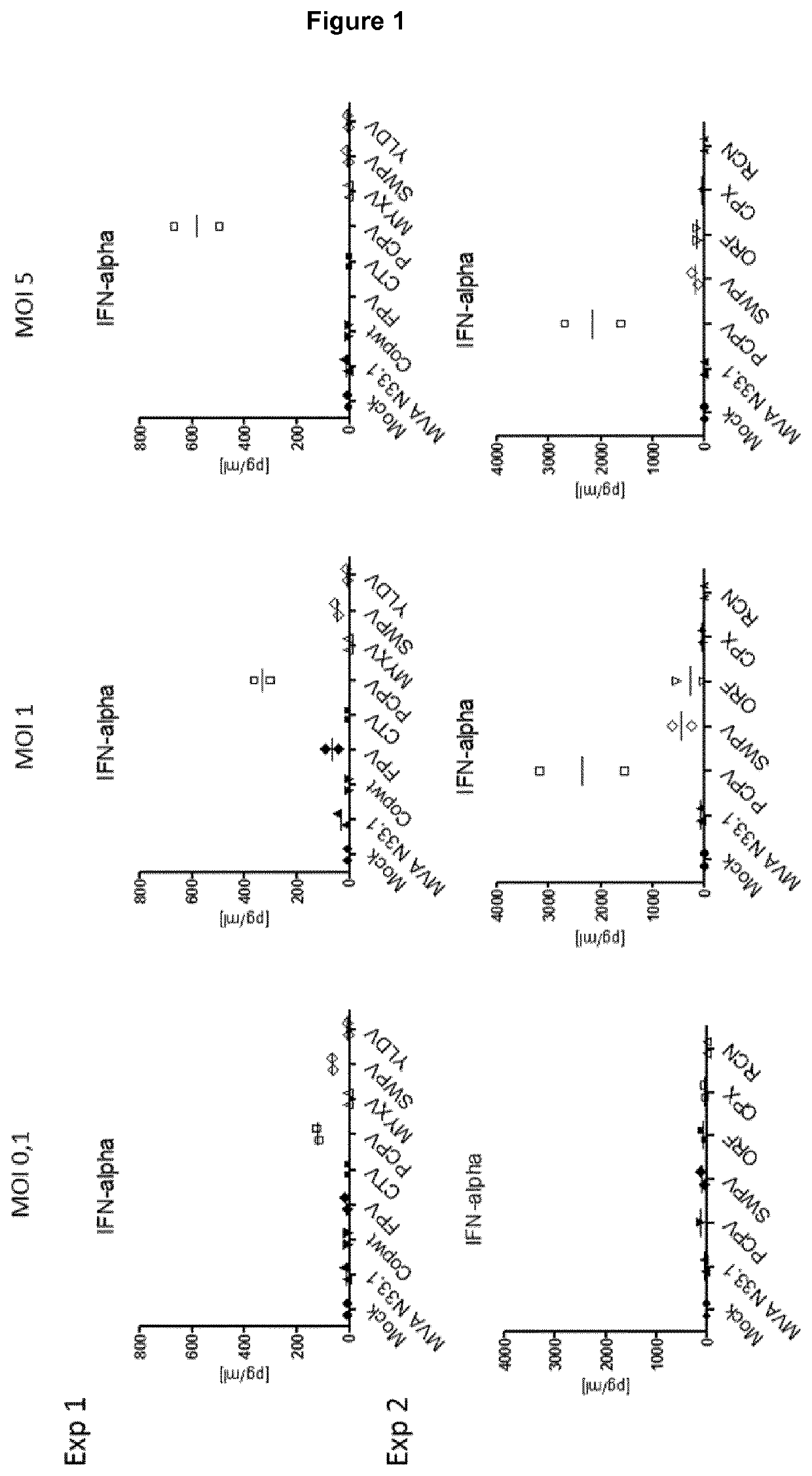
f Secreted Cytokines / Chemokines in Infected Human Peripheral Blood Mononuclear Cells (PBMCs)
[0175]Human peripheral blood mononuclear cells (PBMCs) were isolated from whole human blood of two healthy donors (EFS, Etablissement de Strasbourg; donor A and B). PBMCs were isolated by density gradient centrifugation using Ficoll-Paque PLUS (GE HealthCare) and Blood Separation Tubes (Greiner) and stored in liquid nitrogen.
[0176]Frozen aliquots of PBMCs were thawed in RPMI supplemented with 10% Fetal calf serum (FSC) and dispatched in 24 well plates (5·105 cells in 500 μL / well). After 5 to 6 hours, cells were infected with the different viruses (see below) at multiplicities of infection (MOI) between 10−3 and 10. After overnight incubation (16 hours), cells were scraped, cells and supernatant were transferred in Eppendorf tubes and centrifuged 10 min at 300 g. Supernatant was isolated and frozen at −80° C. Cytokine and chemokine profiles were quantified by a Multiplex approach in the supern...
example 2
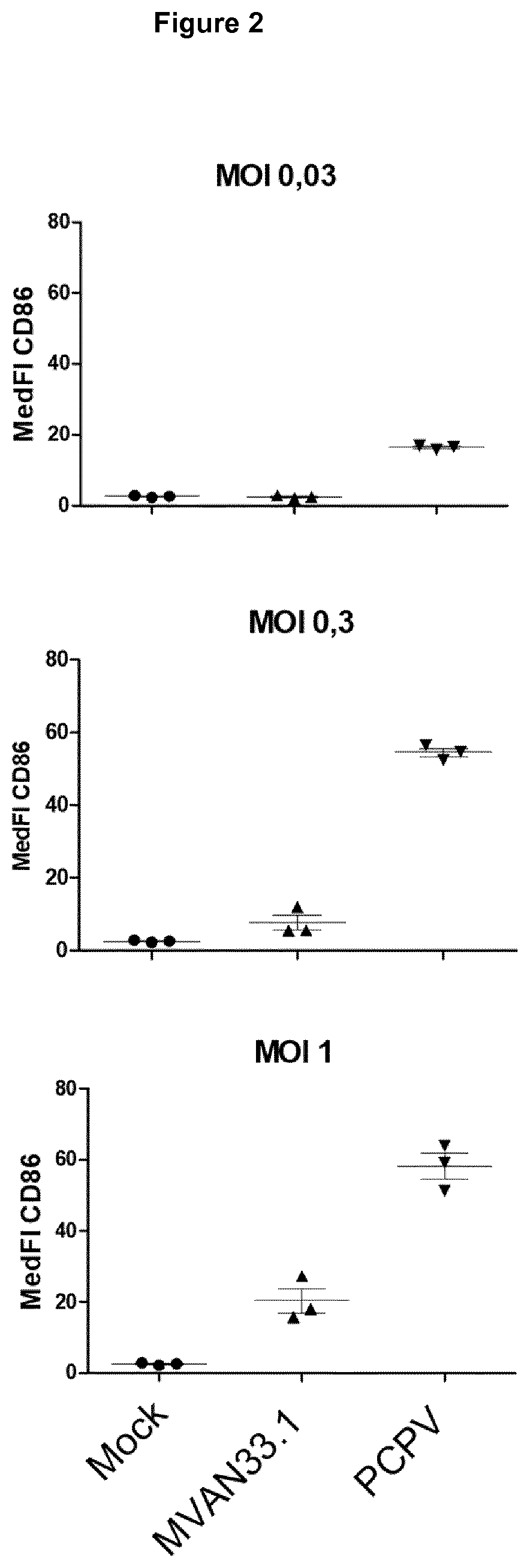
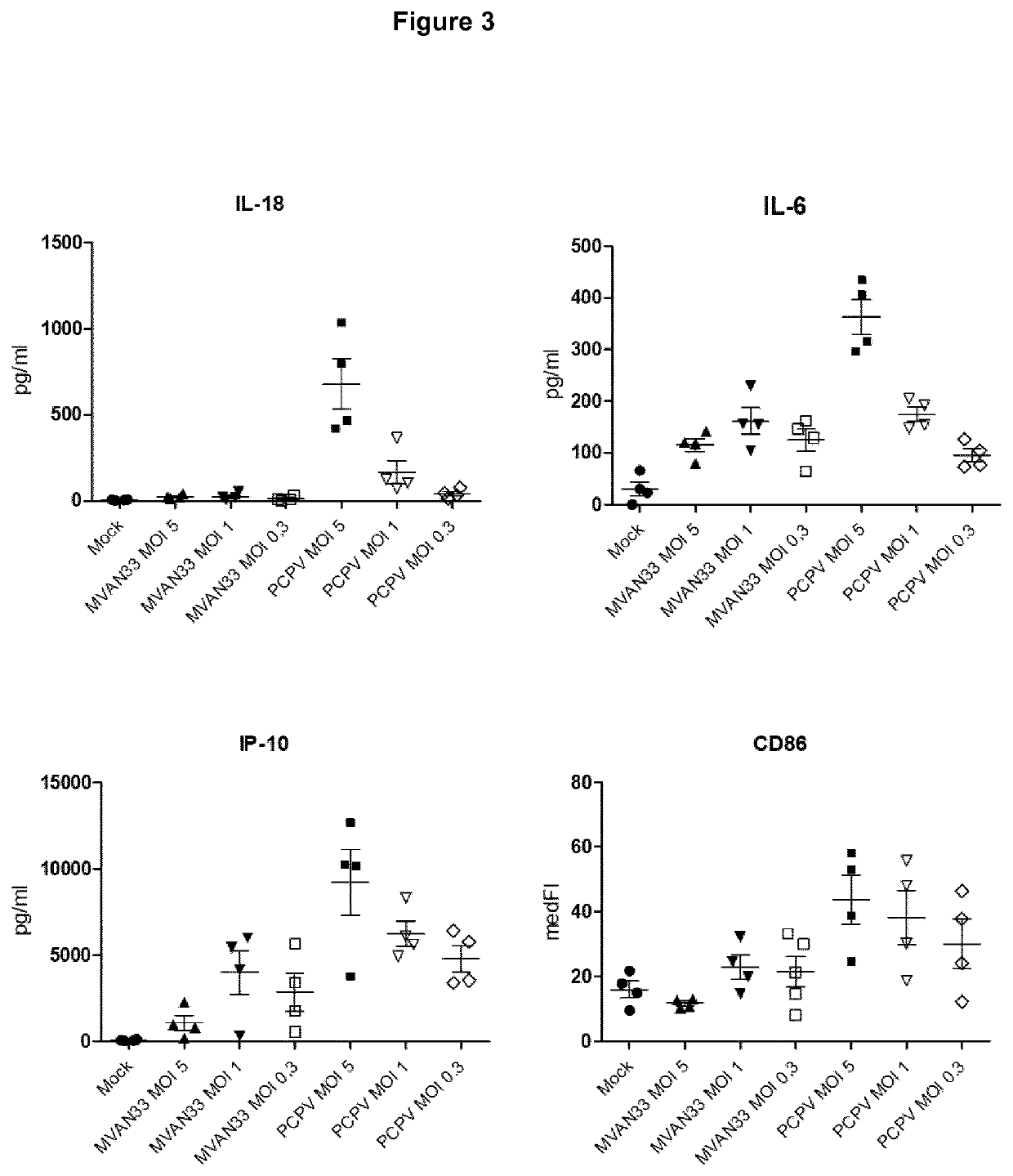
PCPV on Human Monocyte-Derived Dendritic Cells, M2 Macrophages and Myeloid-Derived Suppressor Cells (MDSCs)
[0180]Type I interferons like IFN-alpha are key players in immunotherapy of cancer as well as altered immunosuppressive tumor environment and improved adaptive anti-tumor responses against vaccine encoded and / or tumor-presented antigens as described e.g. by Parker et al. (2016, Nat Rev Cancer 16(3): 131-44) and Zitvogel et al. (2015, Nat Rev Immunol. 15(7): 405-14) To address these aspects, we looked in vitro in human primary immune cells at the activation of human monocyte-derived dendritic cells (moDC) and at the effect on immunosuppressive cell populations (re-programming of immunosuppressive cells like M2 macrophages and MDSCs) upon PCPV treatment. Preclinical studies on innate and adaptive immunity were also probed in murine syngeneic tumor models.
[0181]Stimulation of moDCs.
[0182]To probe the activation of antigen-presenting cells (APCs), we worked with monocyte-derived de...
example 3
ion of Recombinant PCPV and Anti-Tumor Properties
[0192]The interest for PCPV as viral backbone was evaluated by generating recombinant PCPV encoding tumor antigens and testing them in tumor control experiments.
[0193]Recombinant PCPV vectors were constructed from the wild-type strain TJS (ATTC, VR-634). PCPV, as all the Parapoxvirus, lack genes present or conserved in other poxviruses. These comprises homologues of most poxviral genes likely involved in nucleotide metabolism, including homologues of ribonucleotide reductase (RR), thymidine kinase (TK), guanylate kinase and thymidylate kinase. Therefore, the locus TK generally used for generation of recombinant vaccinia virus could not be used for introduction of recombinant gene in PCPV. However, it was shown that recombinant ORF virus could be generated by insertion of the transgene in the non-essential VEGF gene (Rziha et al., 2000, J. Biotechnol. 83(1-2): 137-145). This locus was therefore evaluated for the generation of recombina...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com