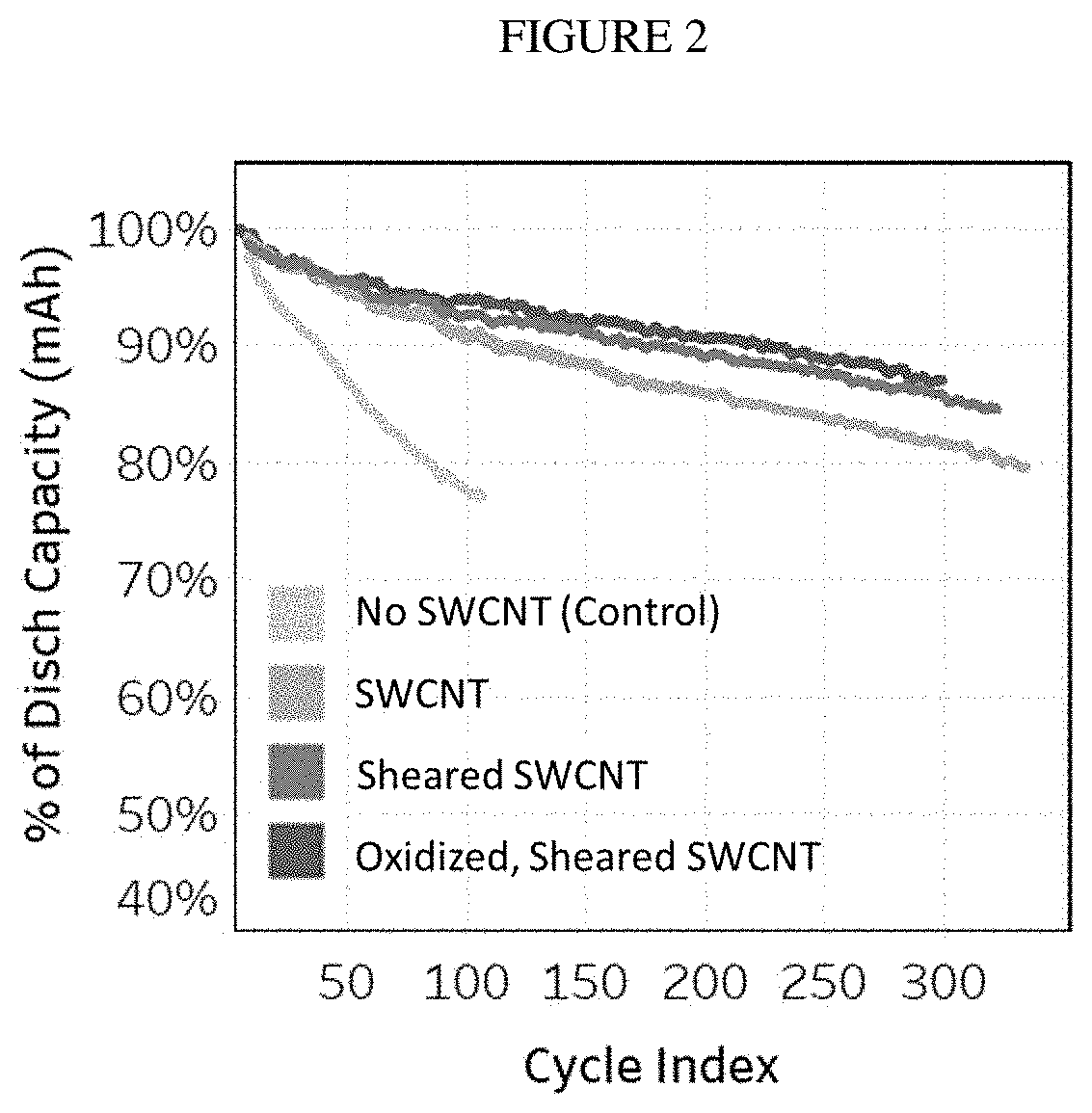
Lithium ion battery using high surface area nanotubes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Tuball™ (OCSiAl)
[0095]Thirty-five grams of >64% nitric acid is heated to 95 degrees C. To the acid, 15 grams of as-received, single-walled carbon nanotubes (Tuball™) are added. The as-received tubes have the morphology of tightly bundled tree-trunks. The mixture of acid and carbon nanotubes are mixed while the solution is kept at about 95 degrees for 5 hours and is labeled “oSWCNT-82-2”. At the end of the reaction period, the oSWCNT 82-2 are filtered to remove the acid and washed with reverse osmosis (RO) water to pH of 3-4. The resulting CNTs were oxidized to about 3.6% and contained about 4.4% metal residue.
[0096]Variations on this process were also conducted using slightly differing parameters as shown below in Table 1:
[0097]Samples oxidized by MAO process: e.g. 35 g HNO3 (65%) / 15 g Tuball™, 95° C. oxidation.
[0098]23.33 g HNO3 (65%)+10.01 g CNT. T=95° C. Initial big plume of NOx at addition of CNT.
TABLE 1Time (hr)T (° C.)% Ox% Res094.21.1121.7195.62.5295.62.44.5395.62.44.9496.22....
example 2
atment of Non-Oxidized and Oxidized OCSiAl Tubes
example 2a
tment of Oxidized OCSiAl Tubes
[0105]Sample volume ˜1200 mL. Use 1.5 L stainless steel container for Rotor / Stator (R / S) work.
[0106]Oxidized OCSiAl ˜0.15%
[0107]Oxidized OCSiAl source: 82-final (pH 3.61, 27.1% solids)
[0108]1200 g×0.15%=1.8 g dry equiv.=6.64 g wetcake. Used 6.65 g wetcake.
[0109]Check viscosity through Rotor Stator R / S as shown below.
T (min)T (° C.)Comments023531Clear liquid droplets on plastic coveringvessel opening. Not viscous941Clear liquid droplets on plasticcovering vessel opening. Notviscous+6.62 g wetcake1550Viscous mixture. Proceed to shearing
Place in Freezer for ˜1.5 hr.
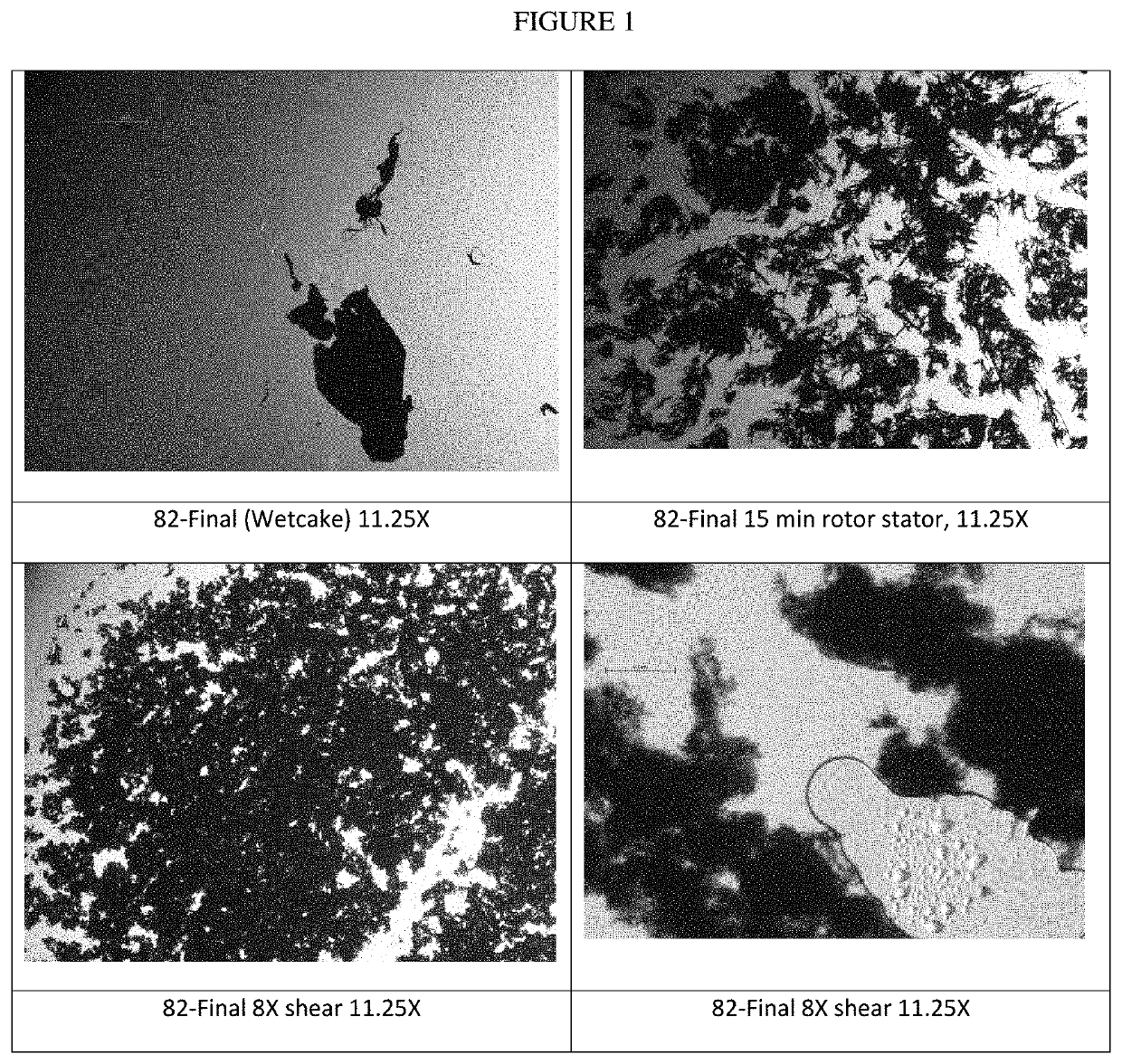
[0110]Shearing
Pass #T (° C.)Comments1251500 psi because noticed some large particlespresent when cleaning the rotor stator236342Place in freezer 45 minutes → 15° C.4315376457511 hr freezer → 25° C.839Sample for optical microscopy
[0111]Sample name 180417-MF-1A (0.26% solids), 180417-MF-1B (0.22% solids) ˜19 g.
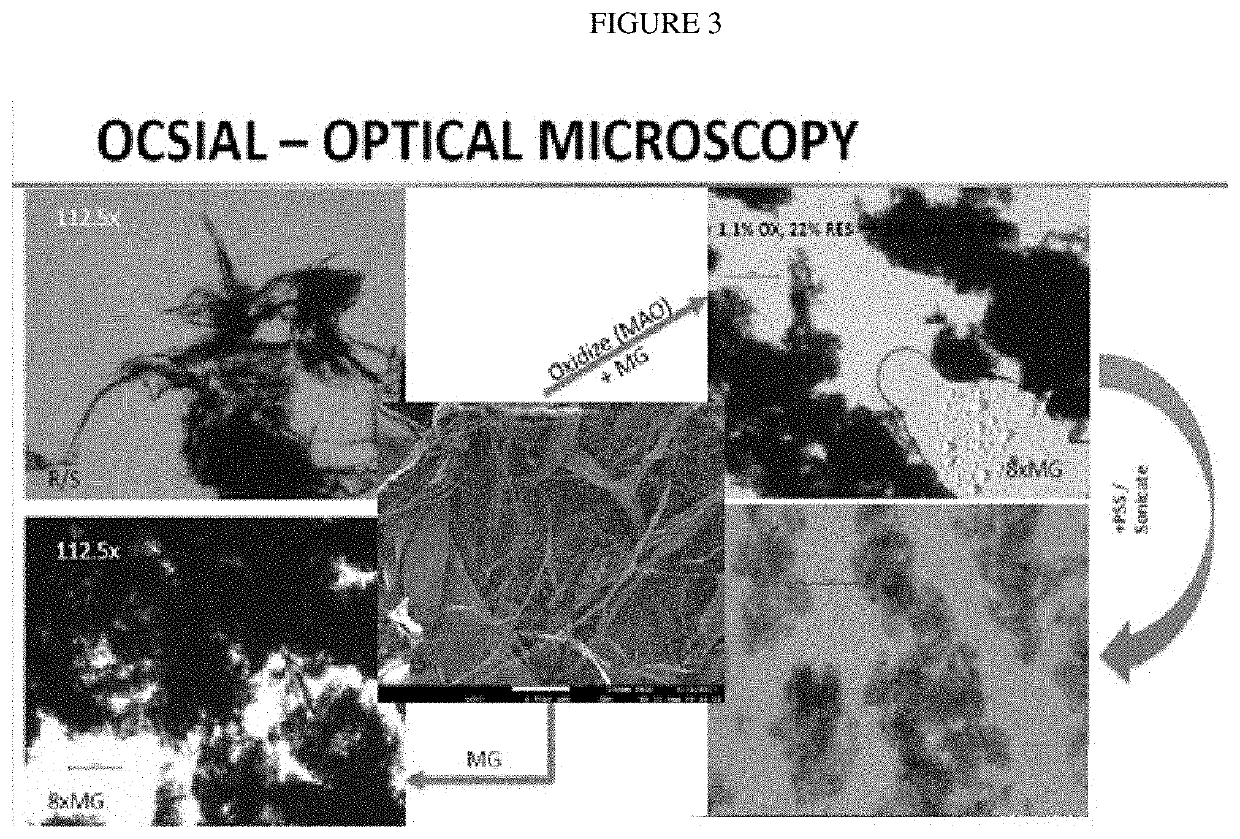
[0112]Optical Microscopy, shown in FIG. 1, shows a progression from wetcake to rotor she...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com