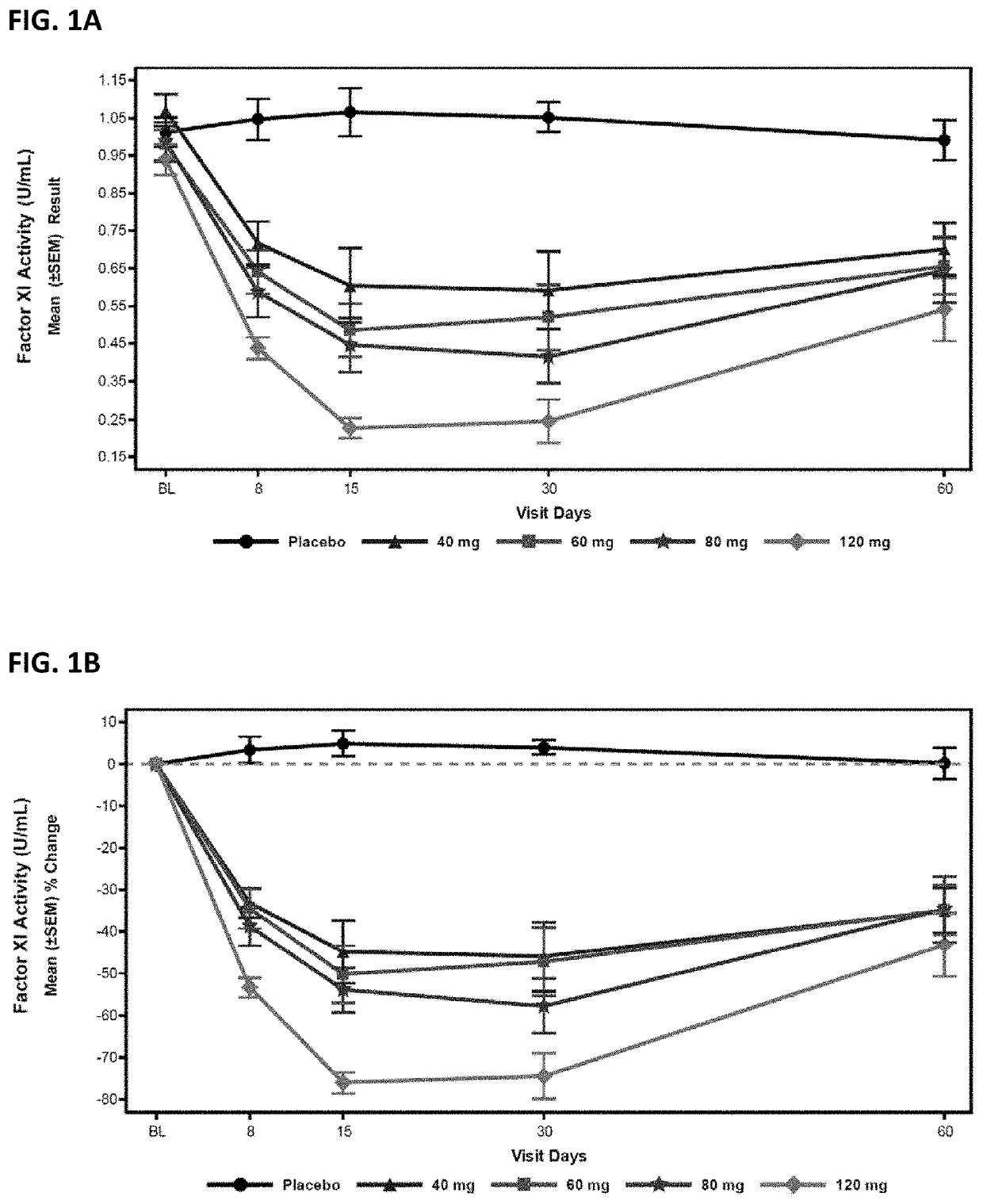
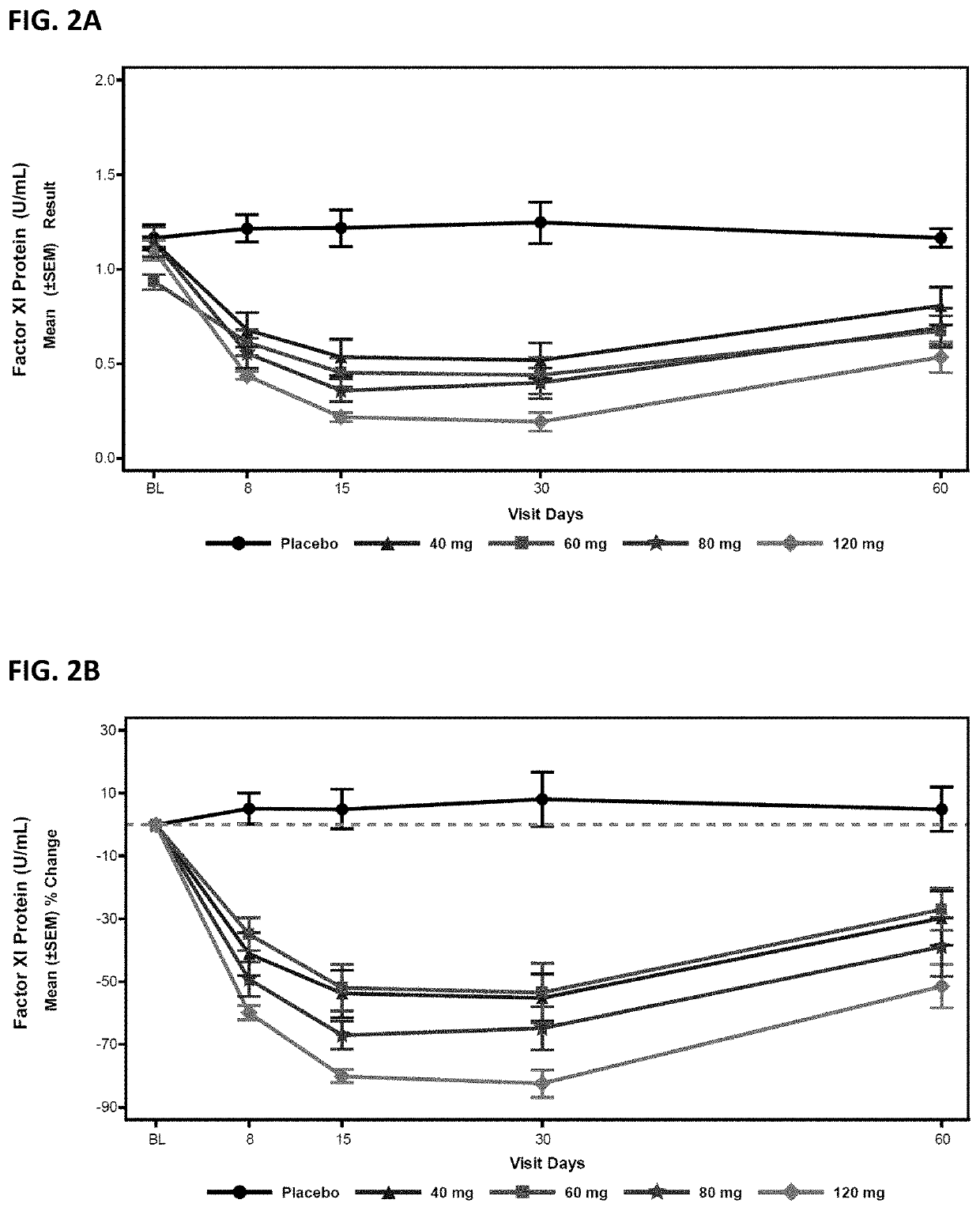
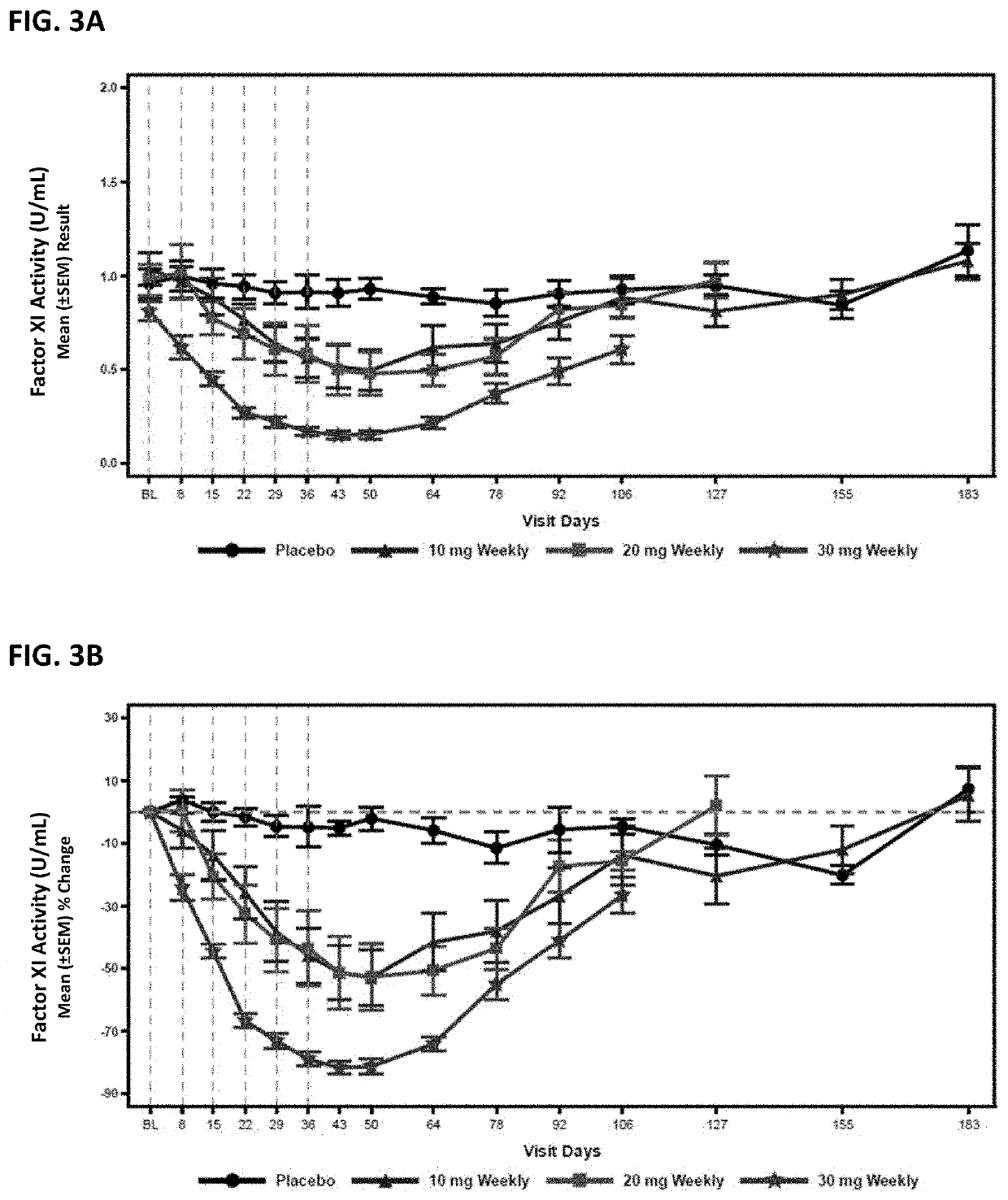
Compounds and methods for reducing fxi expression
a technology of fxi and compound, applied in the field of compound and method for reducing fxi expression, can solve the problems of increased bleeding, increased morbidity, and complicated warfarin drug therapy, and achieve the effects of reducing fxi protein activity, reducing rna, and reducing the amount of fxi protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
f Compound No. 416858 and Compound No. 957943 in Transgenic Mice
[0377]Oligomeric compounds No. 416858 and 957943 were tested in a Factor XI PAC transgenic mouse model which uses bacterial P1 artificial chromosome (PAC) containing the entire WT human Factor XI gene. The mouse model was generated from a human FXI gene fragment containing the entire ˜24 Kb human FXI transgene as well as 9 Kb upstream and 6 Kb downstream. The gene fragment was microinjected into the pronucleus of fertilized mouse eggs, and the complete BAC integration of the transgene was confirmed by PCR using human specific primer probe sets. The established founder has predominate liver expression of human FXI RNA and circulating human FXI in plasma.
TABLE 1Oligomeric compounds complementary tohuman FXI5′ChemistrySEQCompoundModi-NotationIDNumberfication(5′ to 3′)NO:416858noneAesmesGesGesmCesAds3TdsTdsGdsGdsTdsGdsmCdsAdsmCdsAesGesTesTesTe957943(THA-AesmCeoGeoGeomCeoAds3GalNAc3)oTdsTdsGdsGdsTdsGdsmCdsAdsmCdsAeoGeoTesTes...
example 2
ity of Oligomeric Compounds Complementary to Human Factor XI in Cynomolgus Monkeys
[0381]Compound No. 416858 was administered to cynomolgus monkeys at 4, 8, 12, and 40 mg / kg / week by subcutaneous injection for 13 weeks, as described in Husam, et. al., “Antisense inhibition of coagulation factor XI prolongs APTT without increased bleeding risk in cynomolgus monkeys”, Blood, 2012, 119: 2401-2408, incorporated by reference herein in its entirety.
[0382]Compound No. 957943 was administered to groups of 14-18 cynomolgus monkeys, half male and half female, at 1, 6, and 25 mg / kg once a month or 1.5 mg / kg weekly by subcutaneous injection. Platelet levels were measured during routine CBC measurements.
TABLE 4Platelet Counts in Cynomolgus Monkeys after Treatmentwith Compound No. 416858, measured at day 93Dose (weekly)none481240(PBS)mg / kgmg / kgmg / kgmg / kgPlatelets (×103 / μL)506516452404357
TABLE 5Platelet Counts in Cynomolgus Monkeys after treatmentwith Compound No. 957943 for up to 87 daysDose1.51625...
example 3
f Compound No. 416858 and Compound No. 957943 In Vitro in HepatoPac® Cells
[0383]The HepatoPac® kit is a commercially-available in vitro liver model system available from BIOIVT that consists of micropatterned hepatocyte “islands” co-cultured with supportive stromal cells. A 96-well HepatoPac plate was equilibrated for 48 hours at 37° C. and 10% CO2 in fresh maintenance medium prior to treatment. Oligomeric compounds were diluted into maintenance medium at 0.0002, 0.0020, 0.0200, 0.2000, 2.0000, or 20.0000 μM for 48 hours. After 48 hours, medium was replaced with fresh maintenance medium without additional compound. Cell lysates were collected at 96 hours post compound addition and analyzed by RT-PCR using primer probe set RTS2966 (forward sequence CAGCCTGGAGCATCGTAACA, designated herein as SEQ ID NO: 7; reverse sequence TTTATCGAGCTTCGTTATTCTGGTT designated herein as SEQ ID NO: 8; probe sequence TTGTCTACTGAAGCACACCCAAACAGGGA designated herein as SEQ ID NO. 9.). IC50 was calculated us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com