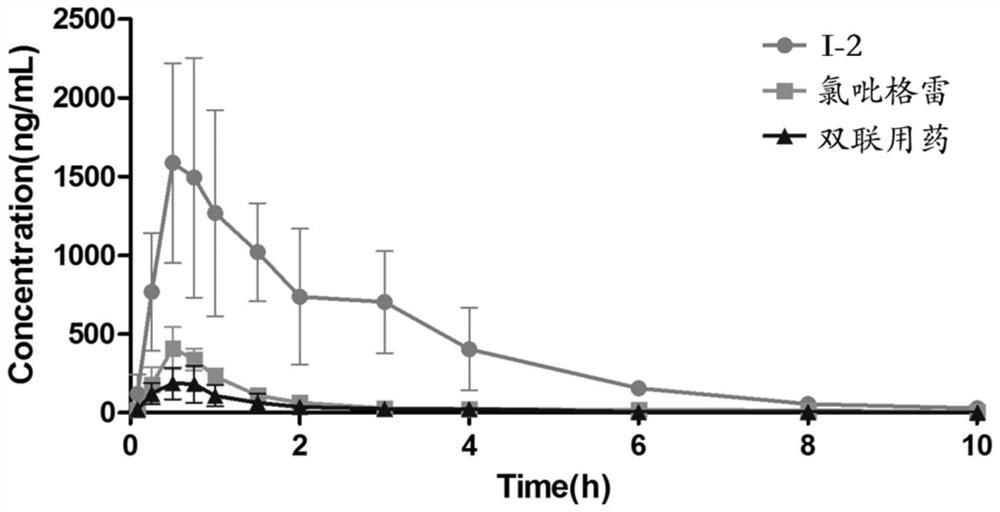
Patents
Literature
44 results about "Hemorrhagic risk" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hemorrhagic stroke is the second most common form of acute strokes. Patients can develop a hemorrhagic stroke due to many risk factors which include hypertension, cerebral amyloid angiopathy, neoplastic diseases and cerebral aneurysms.
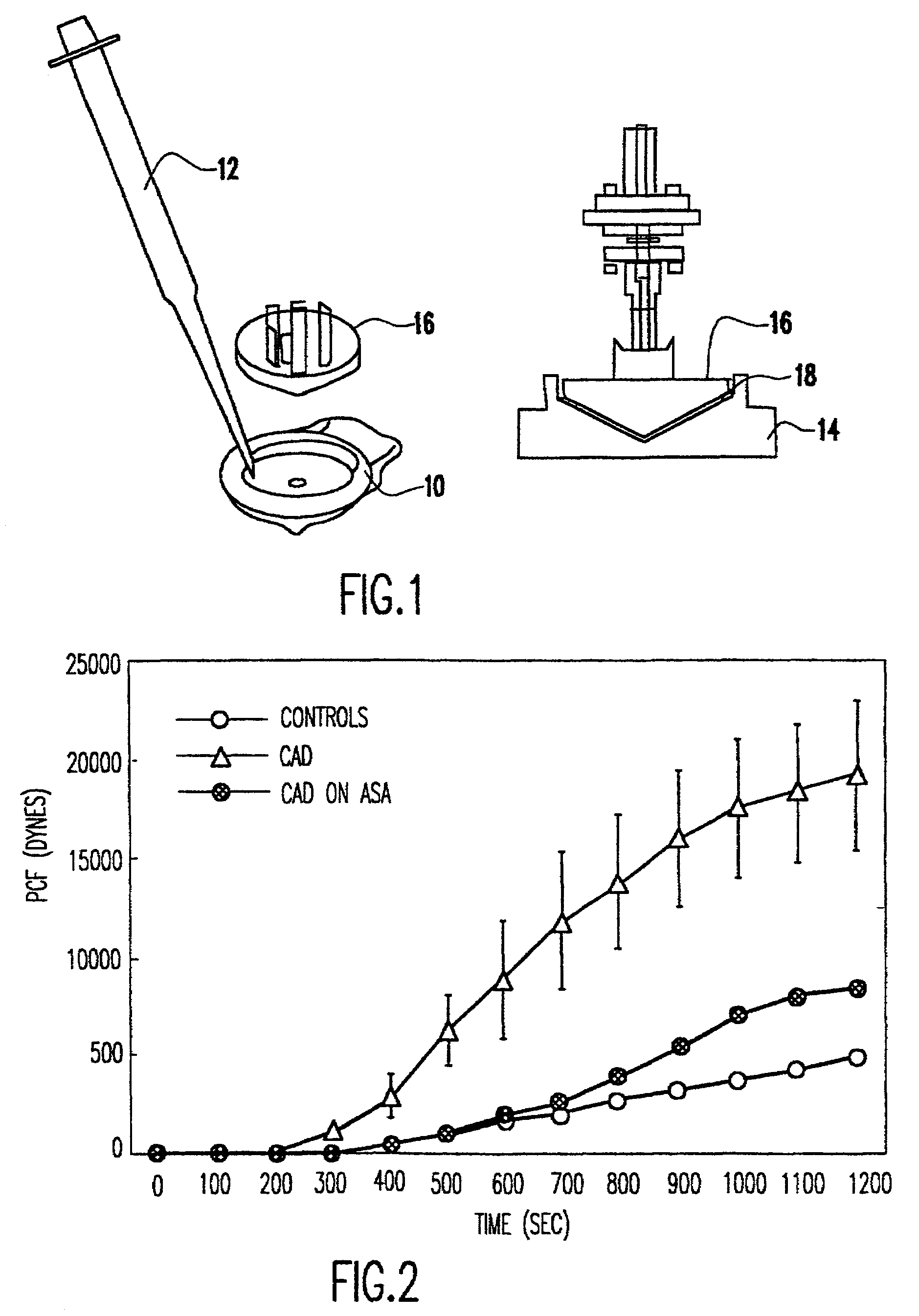
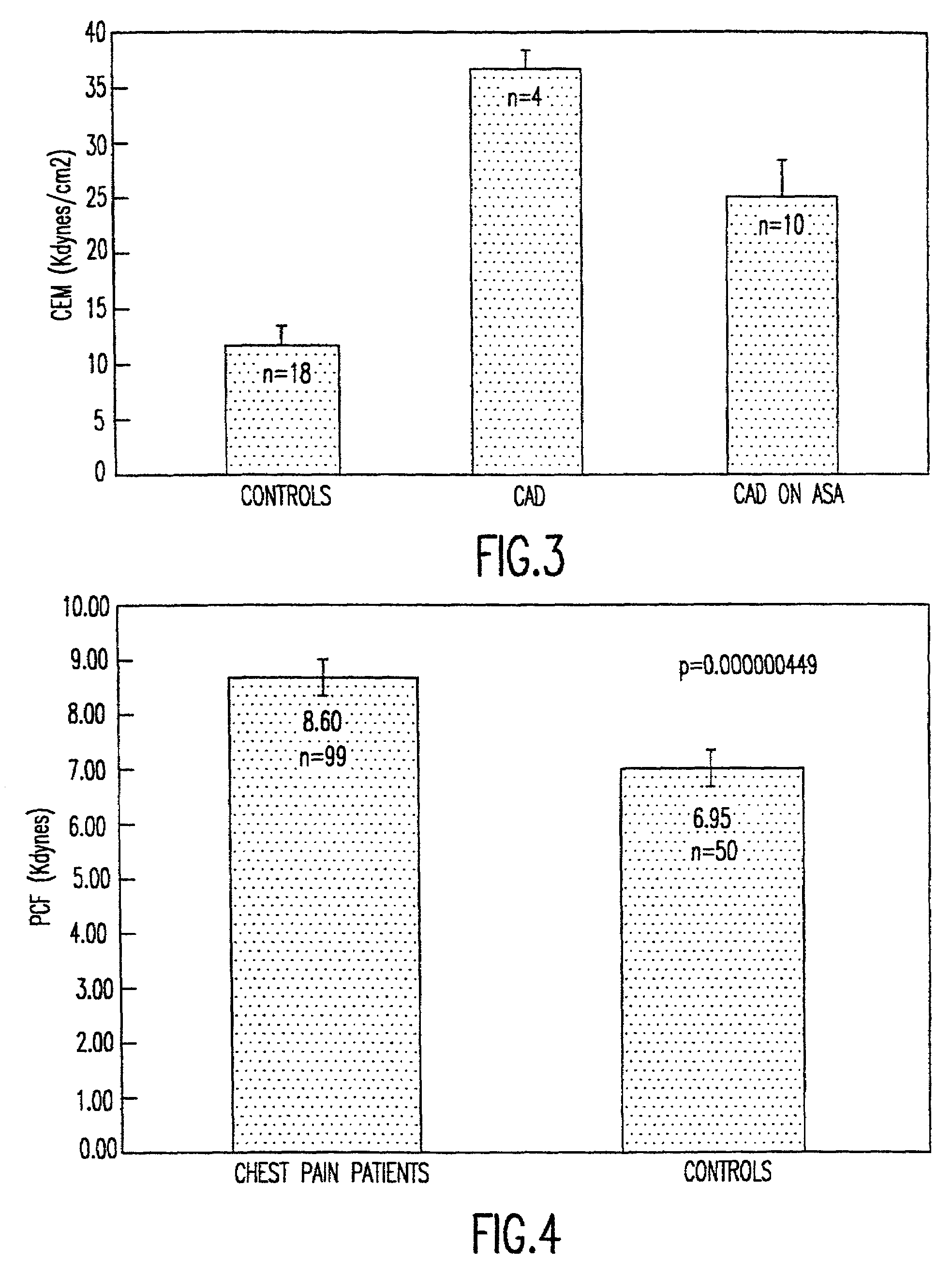
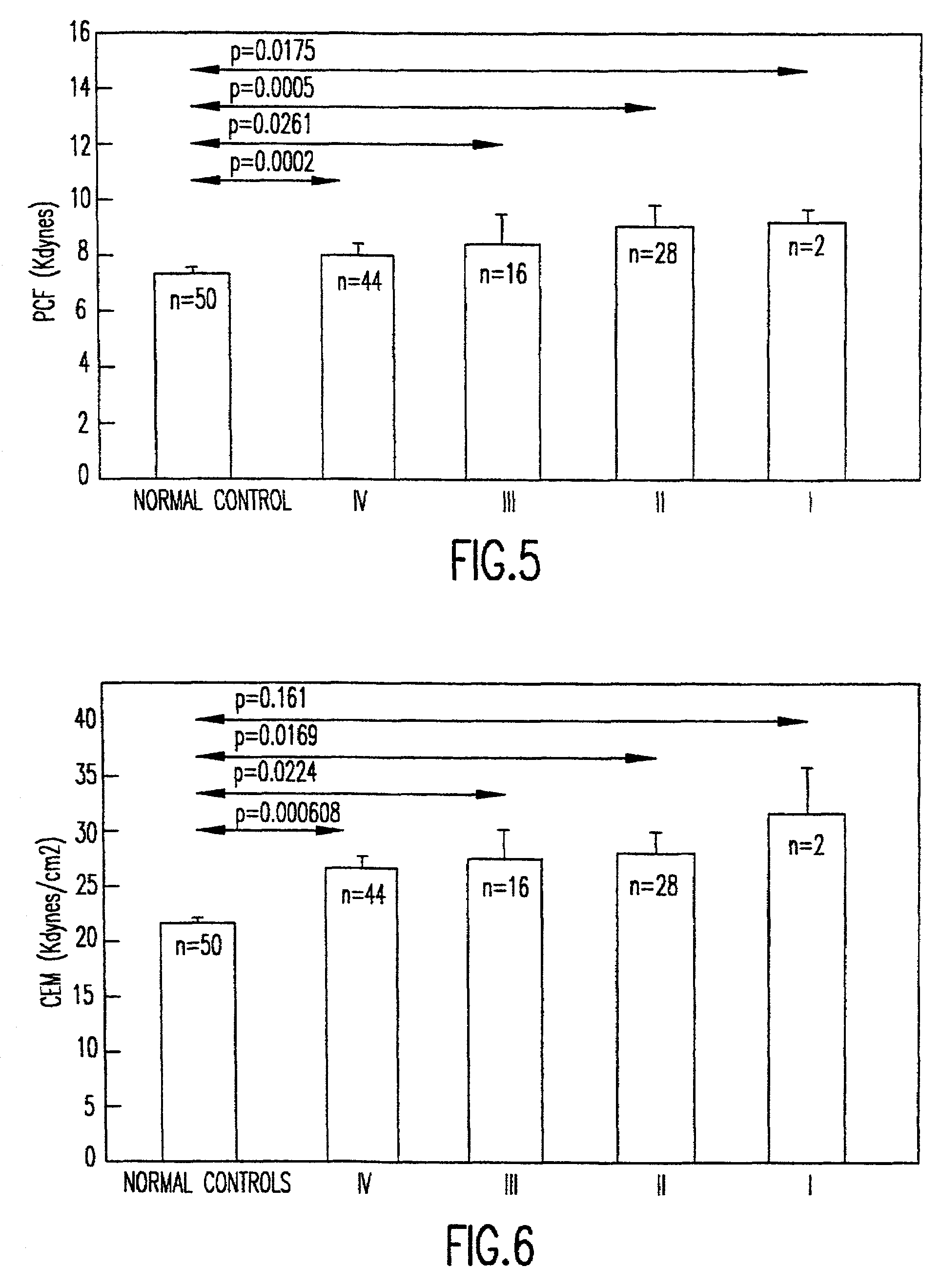
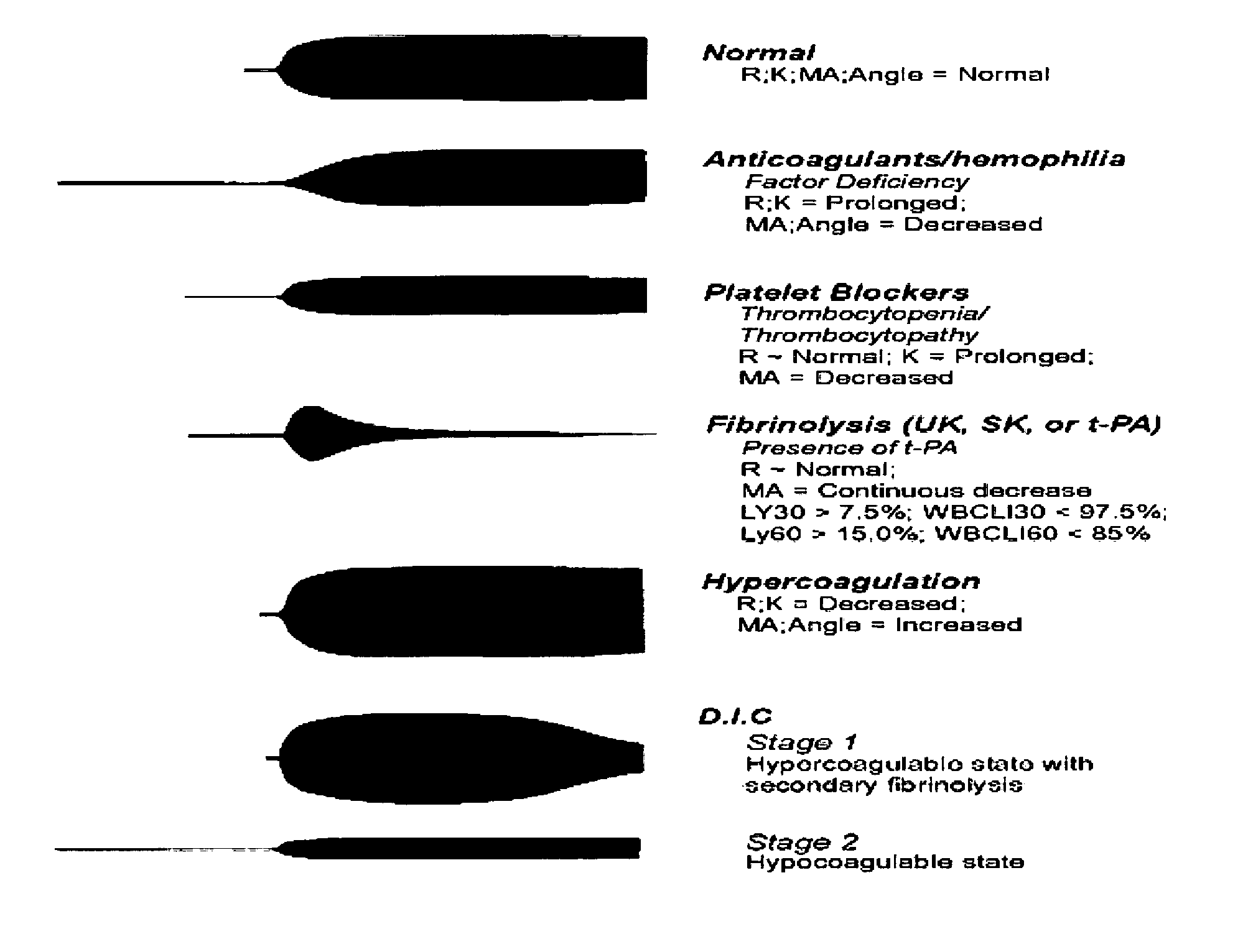
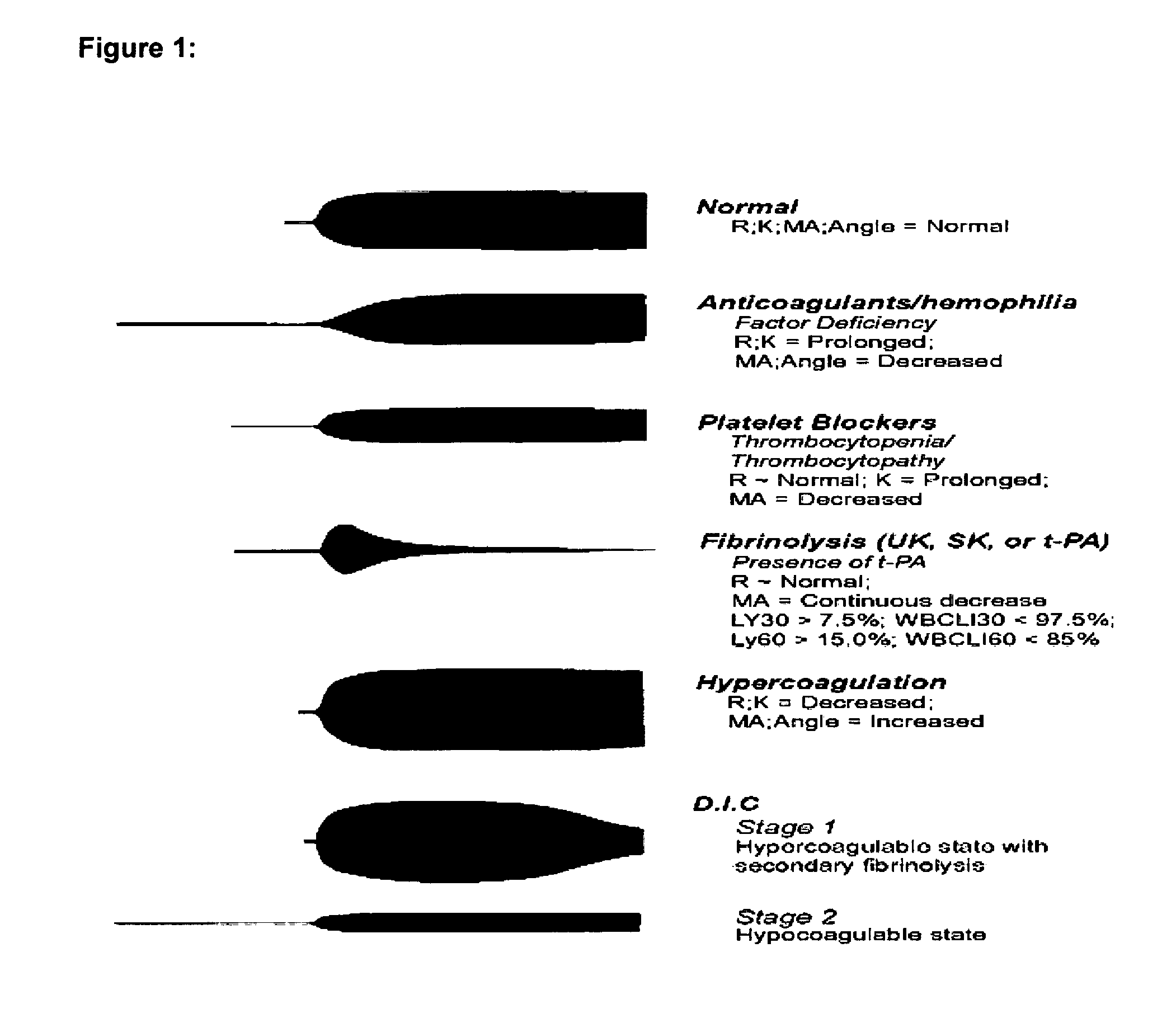
Method of using platelet contractile force and whole blood clot elastic modulus as clinical markers
InactiveUS7192726B1Process is time-consume and expensiveRapid recognition and quantificationMicrobiological testing/measurementDisease diagnosisMedicinePlatelet contraction
Platelet contractile force and / or clot elastic modulus measurements are used to identify patients at risk for atherosclerosis or for bleeding during surgical procedures or other applications. Measurements which are elevated are indicative of atherosclerosis, and measurements which are reduced are indicative of a bleeding risk.
Owner:HEMODYNE
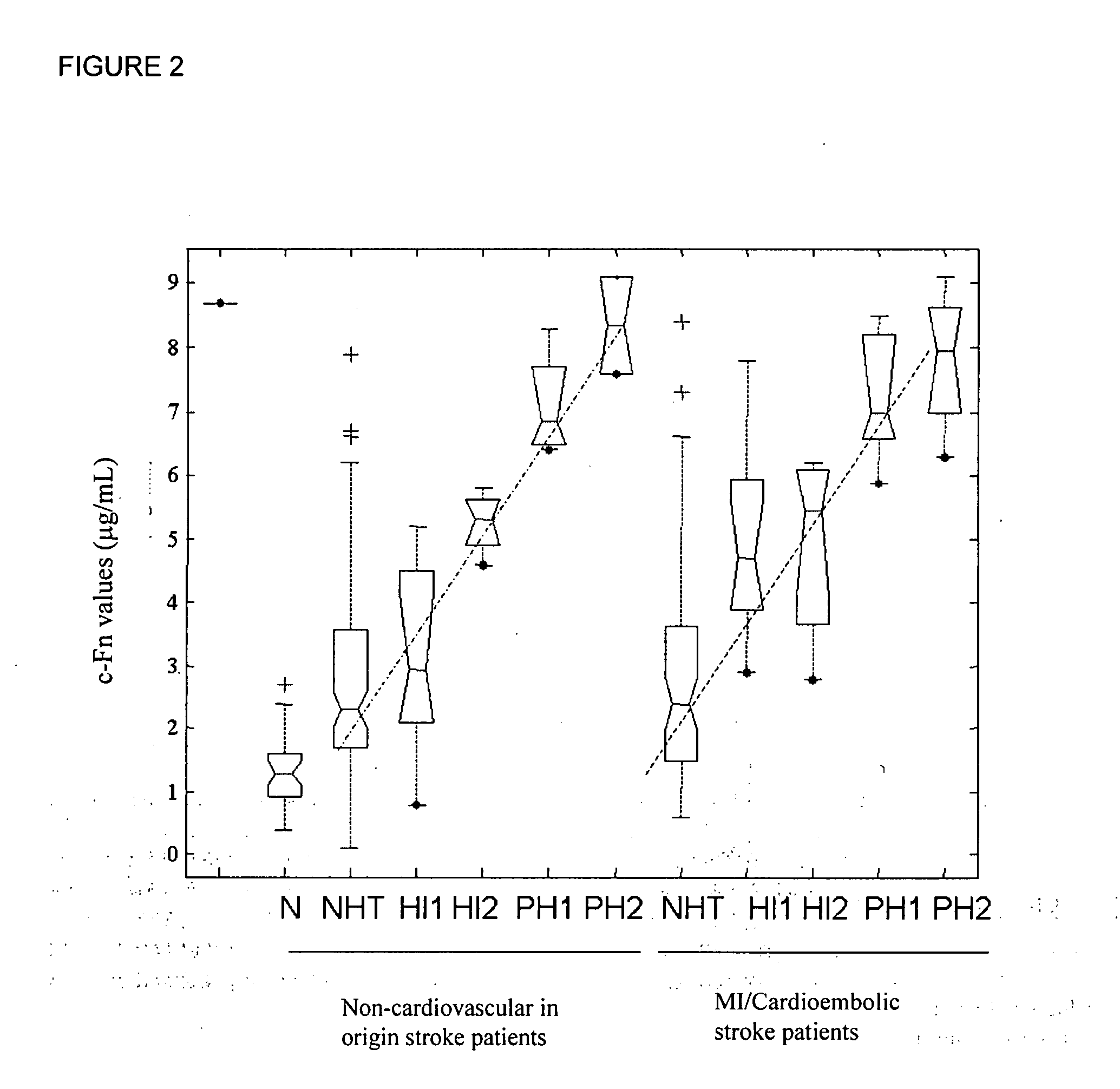
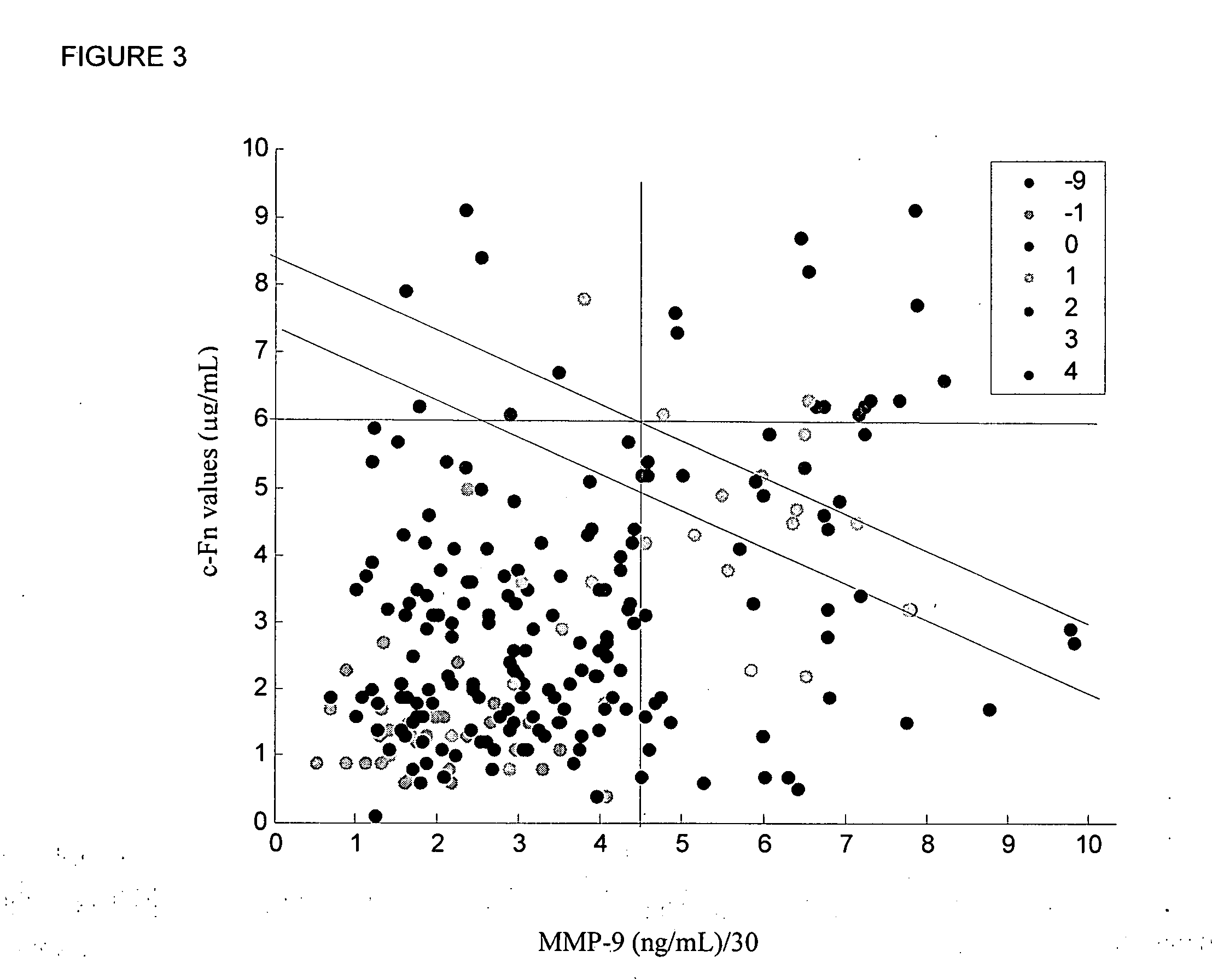
Cellular fibronectin as a diagnostic marker in cardiovascular disease and methods of use thereof
InactiveUS20080010024A1Reduce eliminateProlonged plasma half-lifeDisease diagnosisBiological testingCardiovascular InjuryCardiovascular Disorder
Thrombolytic therapy in the treatment of a cardiovascular event such as myocardial infarction (MI) carries with it a chance of suffering a hemorrhagic incident leading to severe disability and often death. Methods for the evaluation of proper therapy for a specific patient who has suffered a cardiovascular event employ a variety of bio-markers including cellular fibronectin (c-Fn) assembled as a panel for evaluation. Methods are disclosed for selecting markers and correlating their combined levels with a clinical outcome of interest. In various aspects the methods permit early detection of potential bleeding events, determination of the prognosis of a patient presenting cardiovascular damage, and identification of a patient at risk for hemorrhage when given thrombolytic therapy. The disclosed methods provide rapid, sensitive and specific assays to greatly reduce the risk of bleeding or the number of patients that can receive the most beneficial treatment for their cardiovascular event, and to reduce the human and economic costs associated with bleeding following such treatments.
Owner:PREDICTION SCI
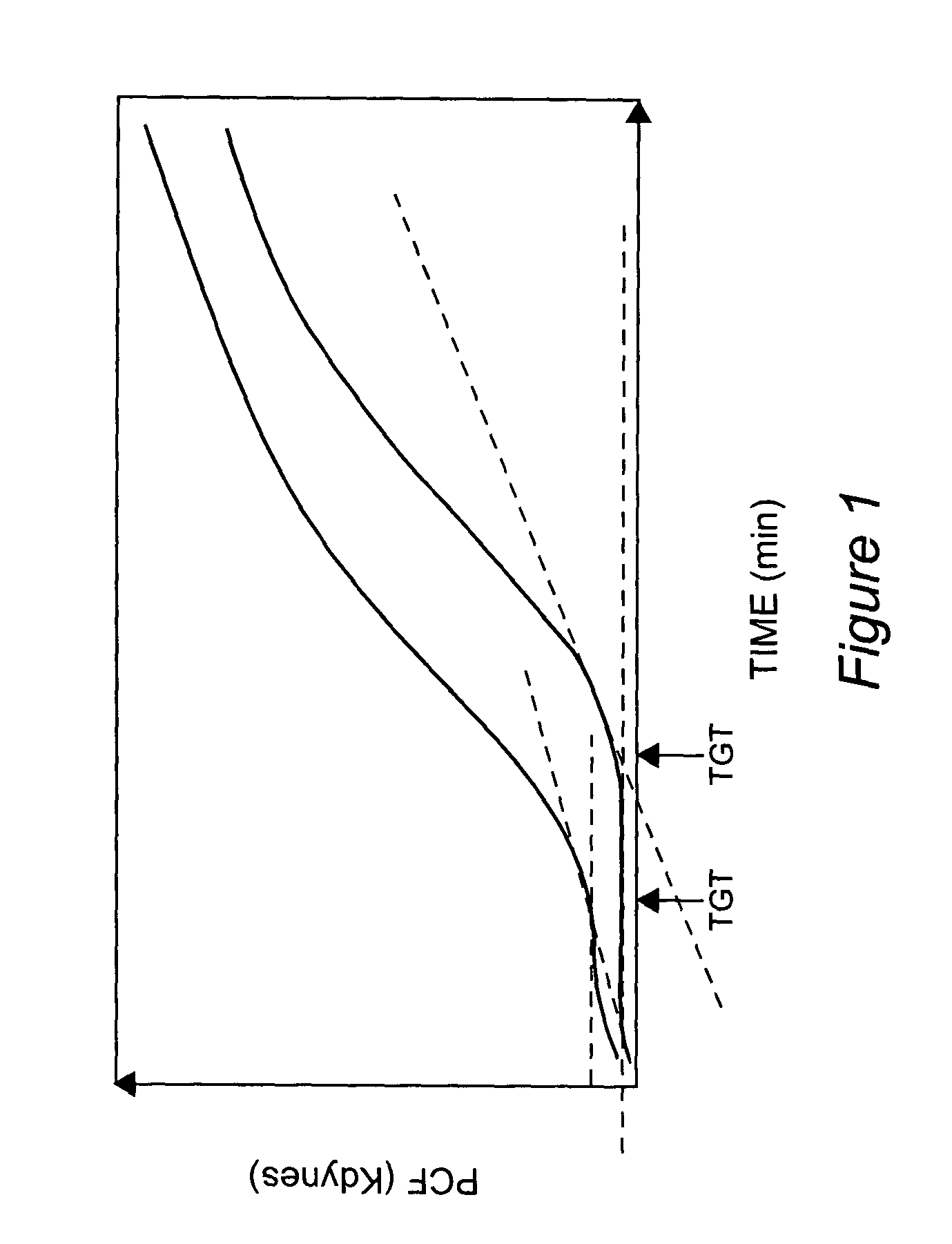
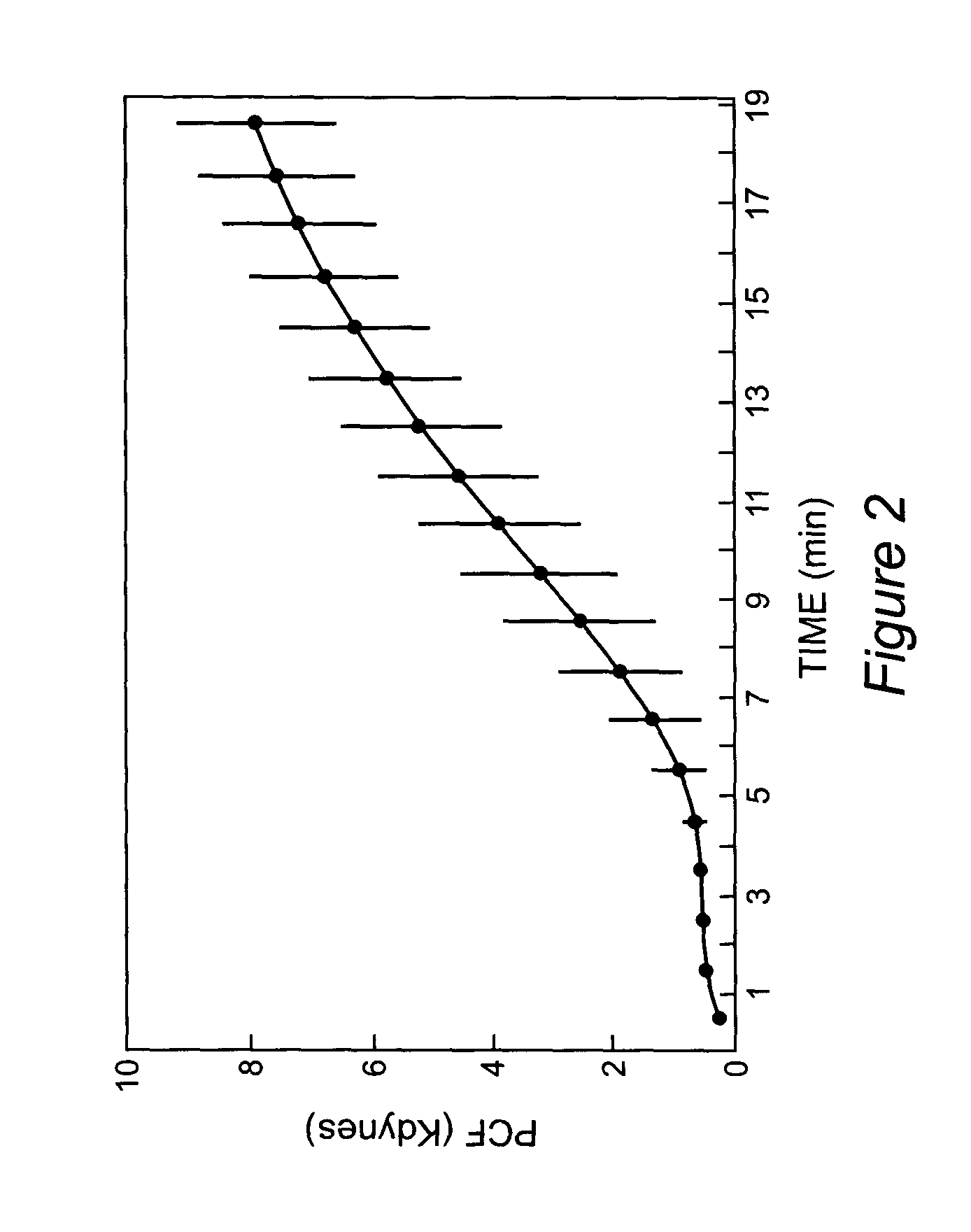
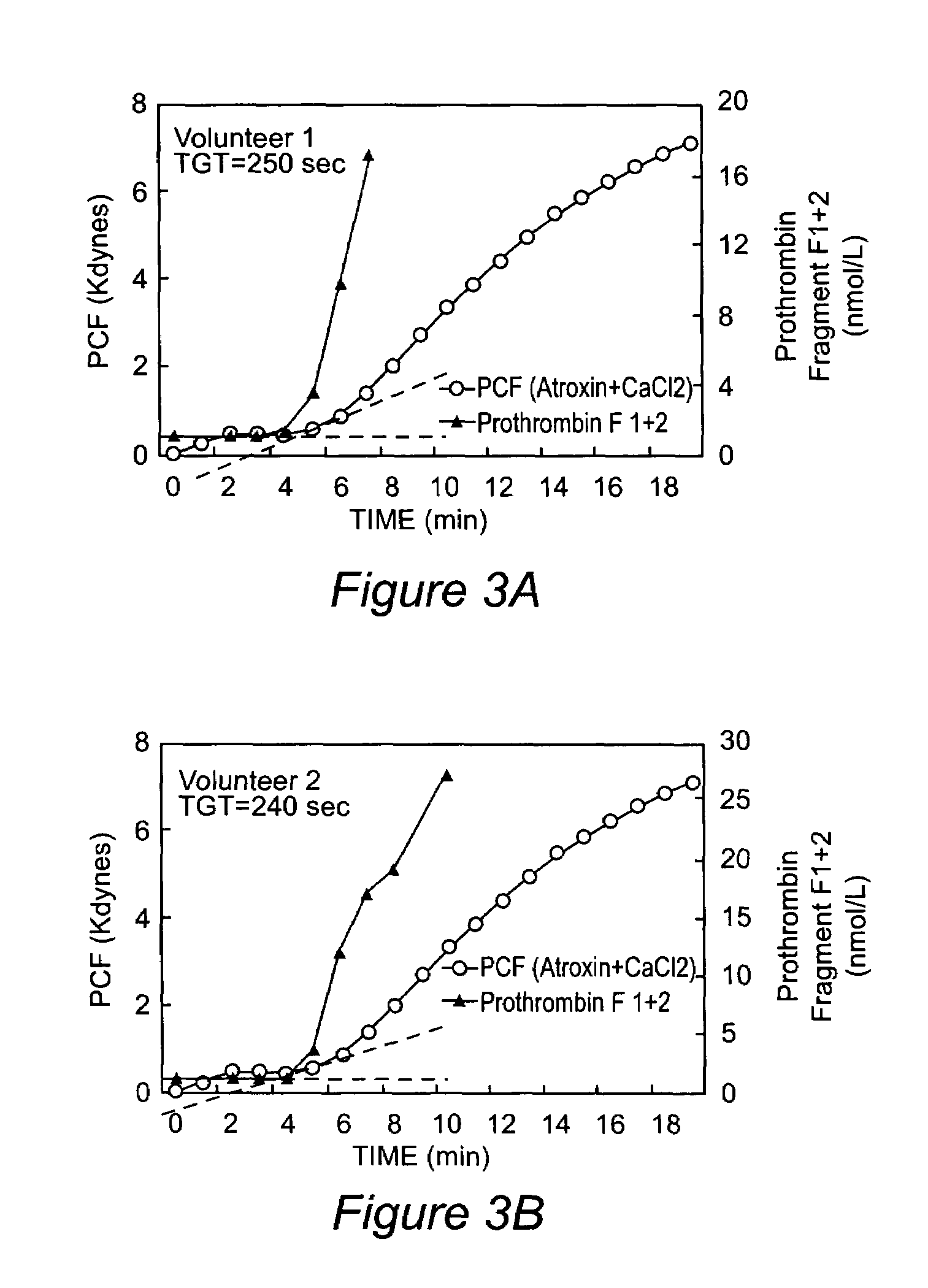
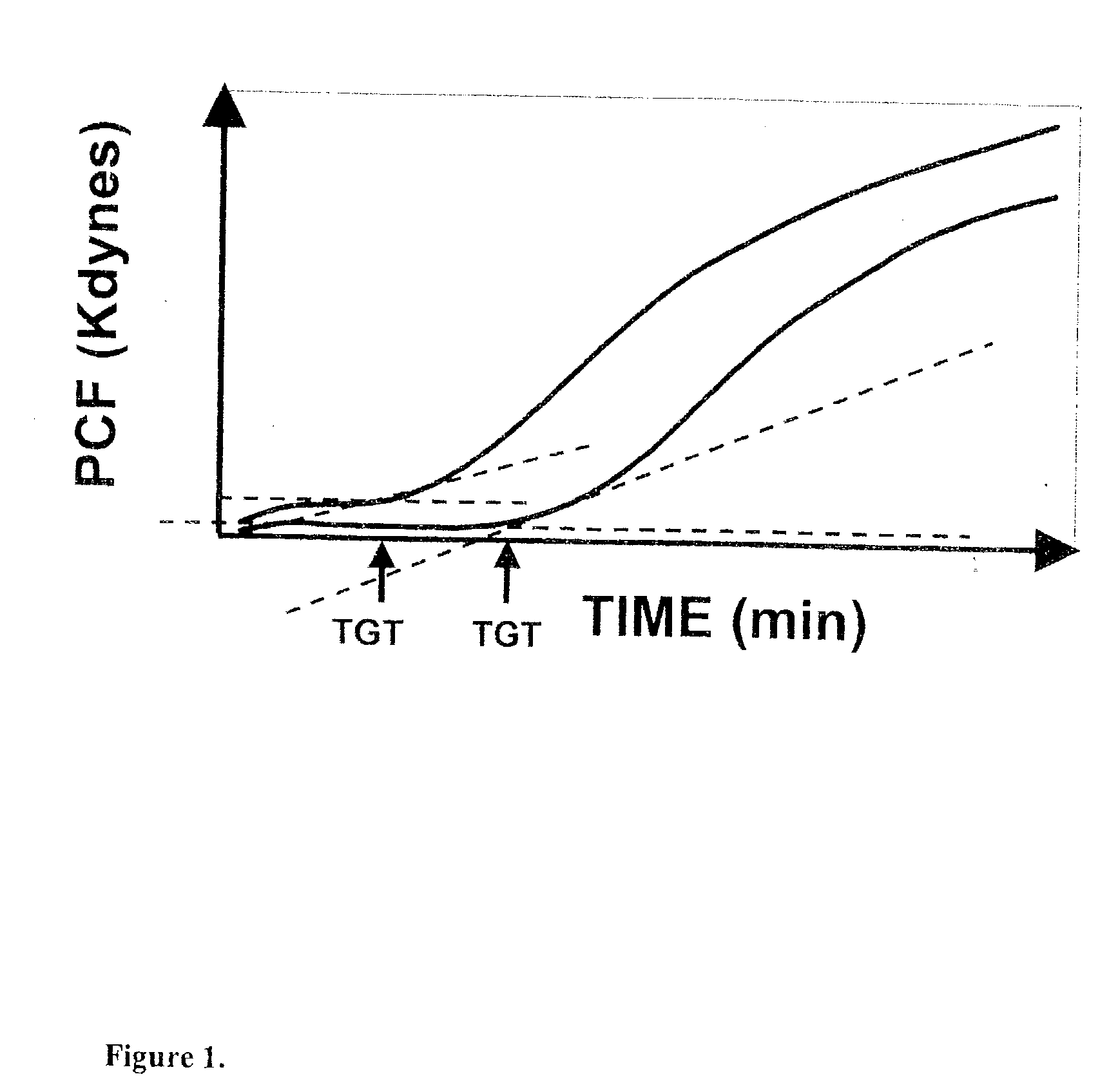
Onset of force development as a marker of thrombin generation
InactiveUS7202048B2Bioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseClotting factor deficiency
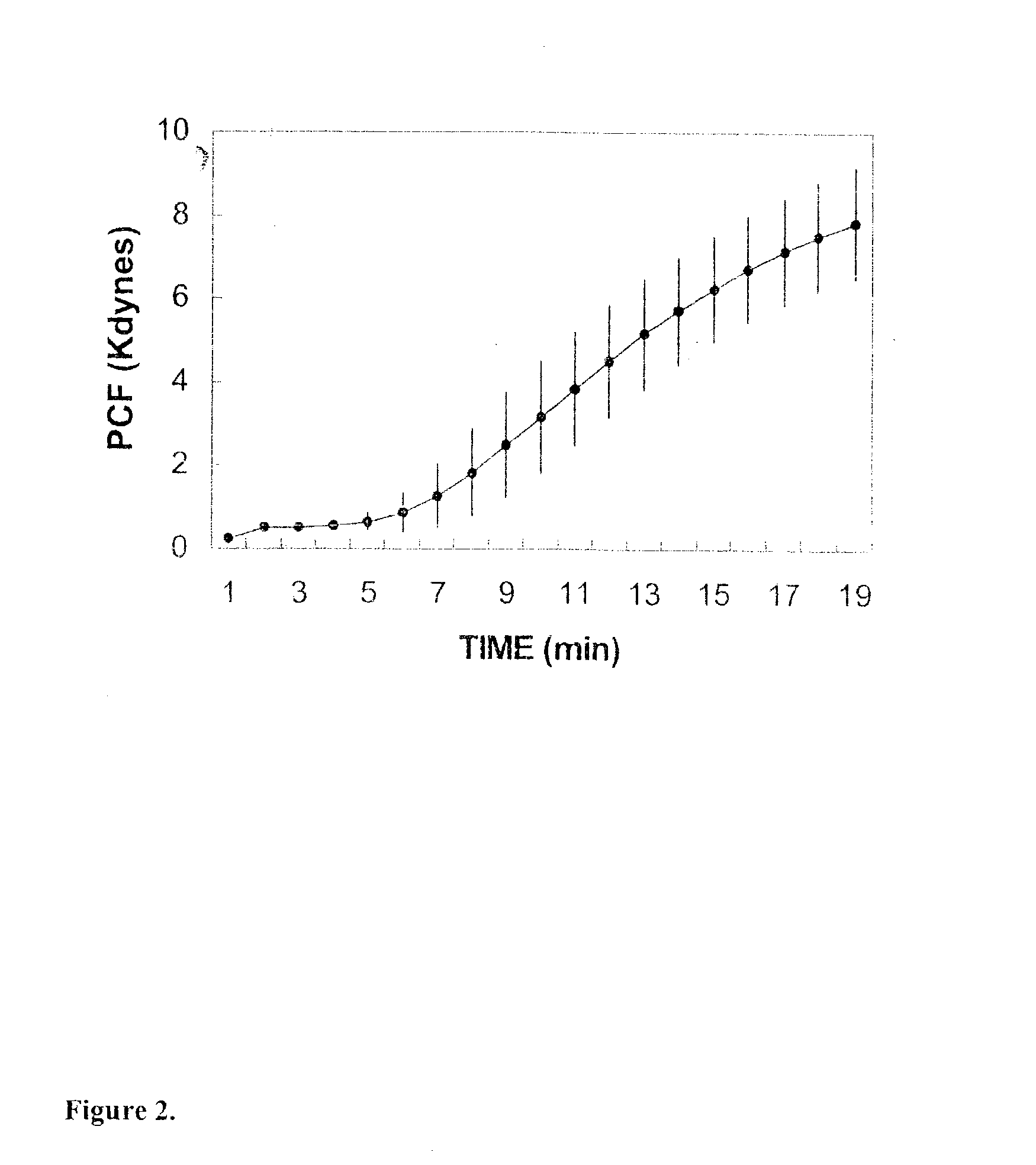
Platelet contractile force (PCF) is used as a surrogate marker of thrombin generation. PCF generation occurs concomitant with the burst of prothrombin fragment F 1+2 release. The time between assay start and PCF onset is identified as the thrombin generation time (TGT), and is used in assessing risk of bleeding, in diagnosing various disorders, and in monitoring the effects of pharmaceutical and other treatments. TGT is prolonged in clotting factor deficiencies and in the presence of direct and indirect thrombin inhibitors. TGT shortens to normal with clotting factor replacement and shortens with administration of rVIIa. TGT is short in thrombophilic states such as coronary artery disease, diabetes and thromboangiitis obliterans and prolongs toward normal with oral and intravenous anticoagulants.
Owner:HEMODYNE
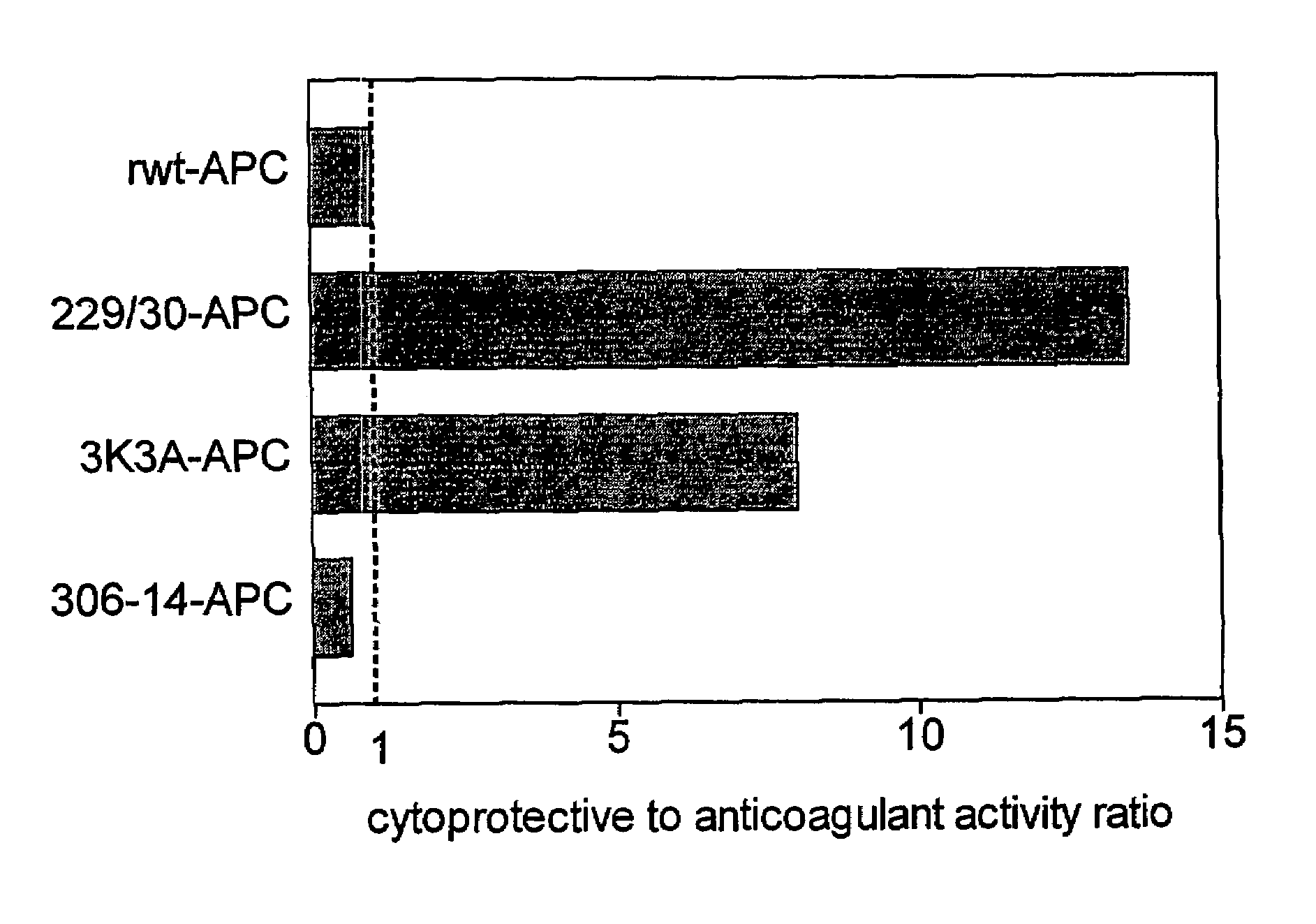
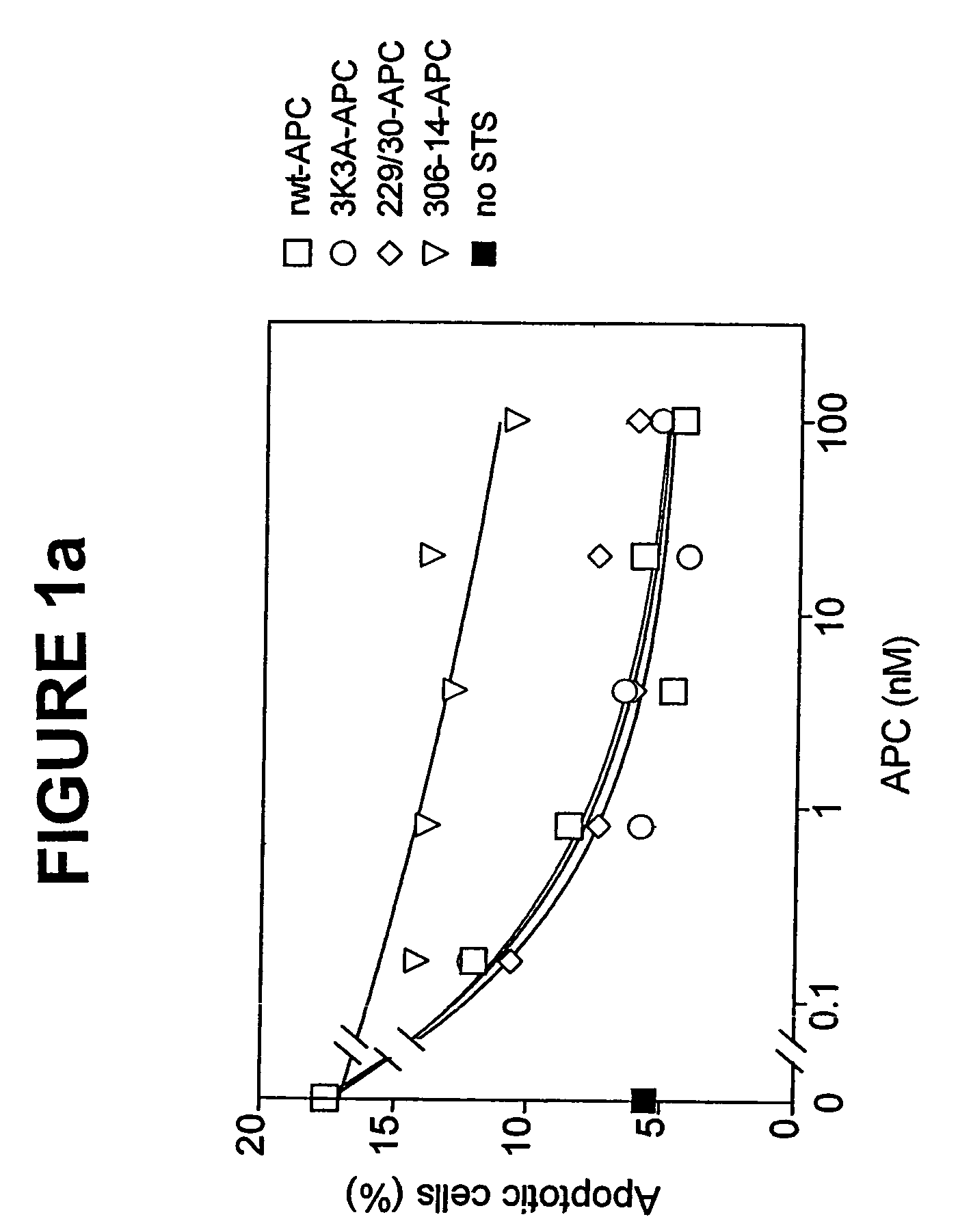
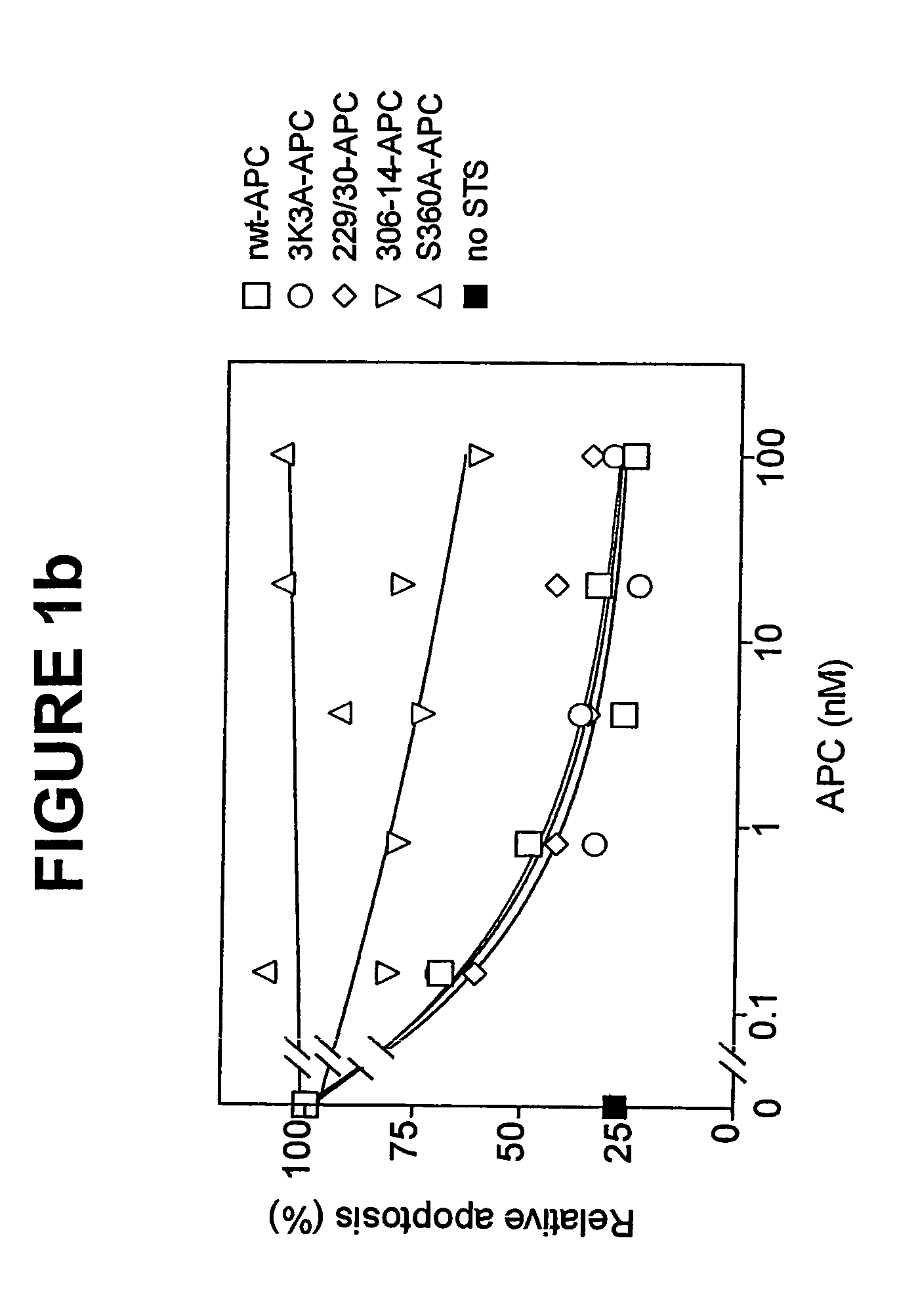
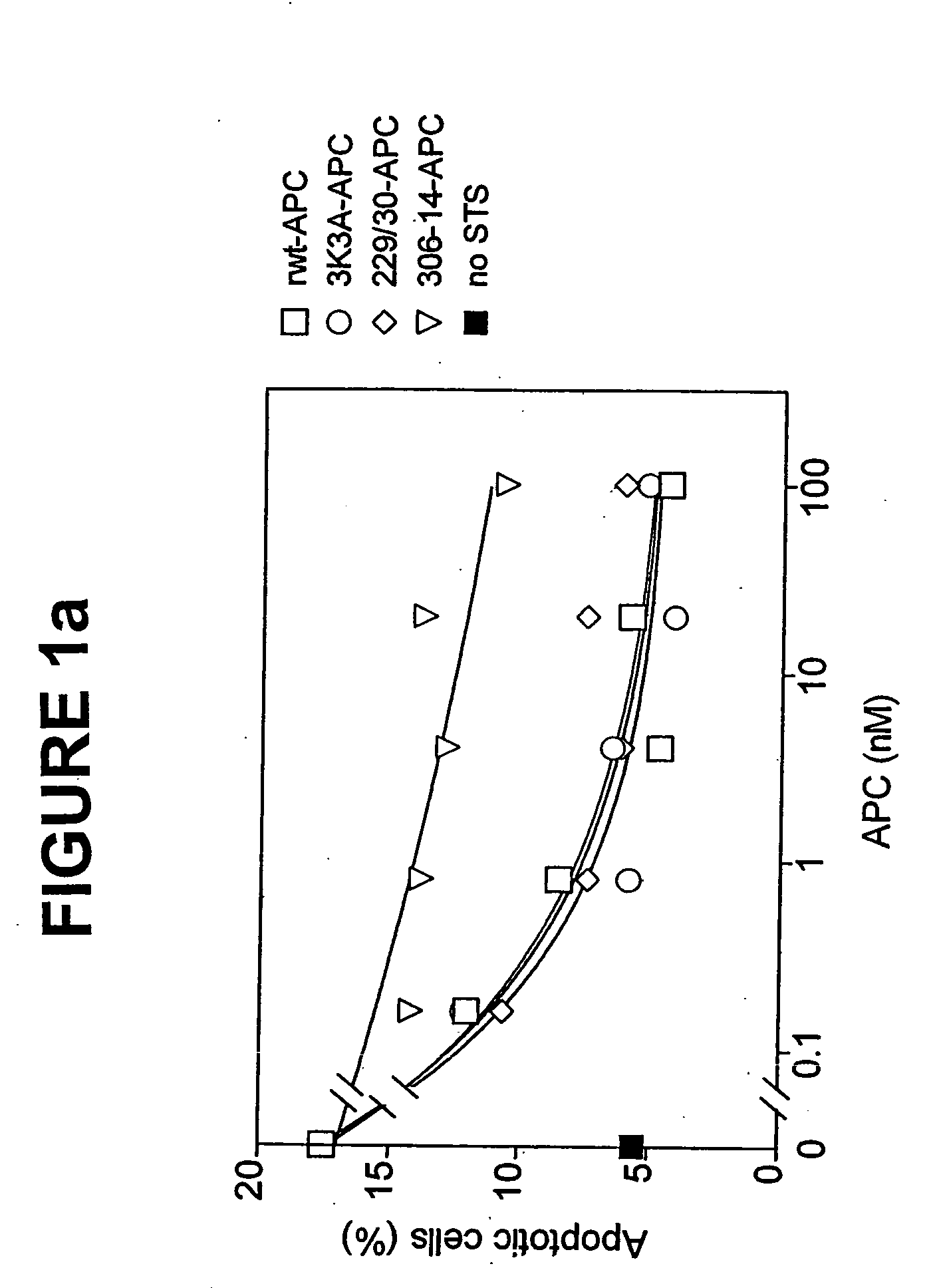
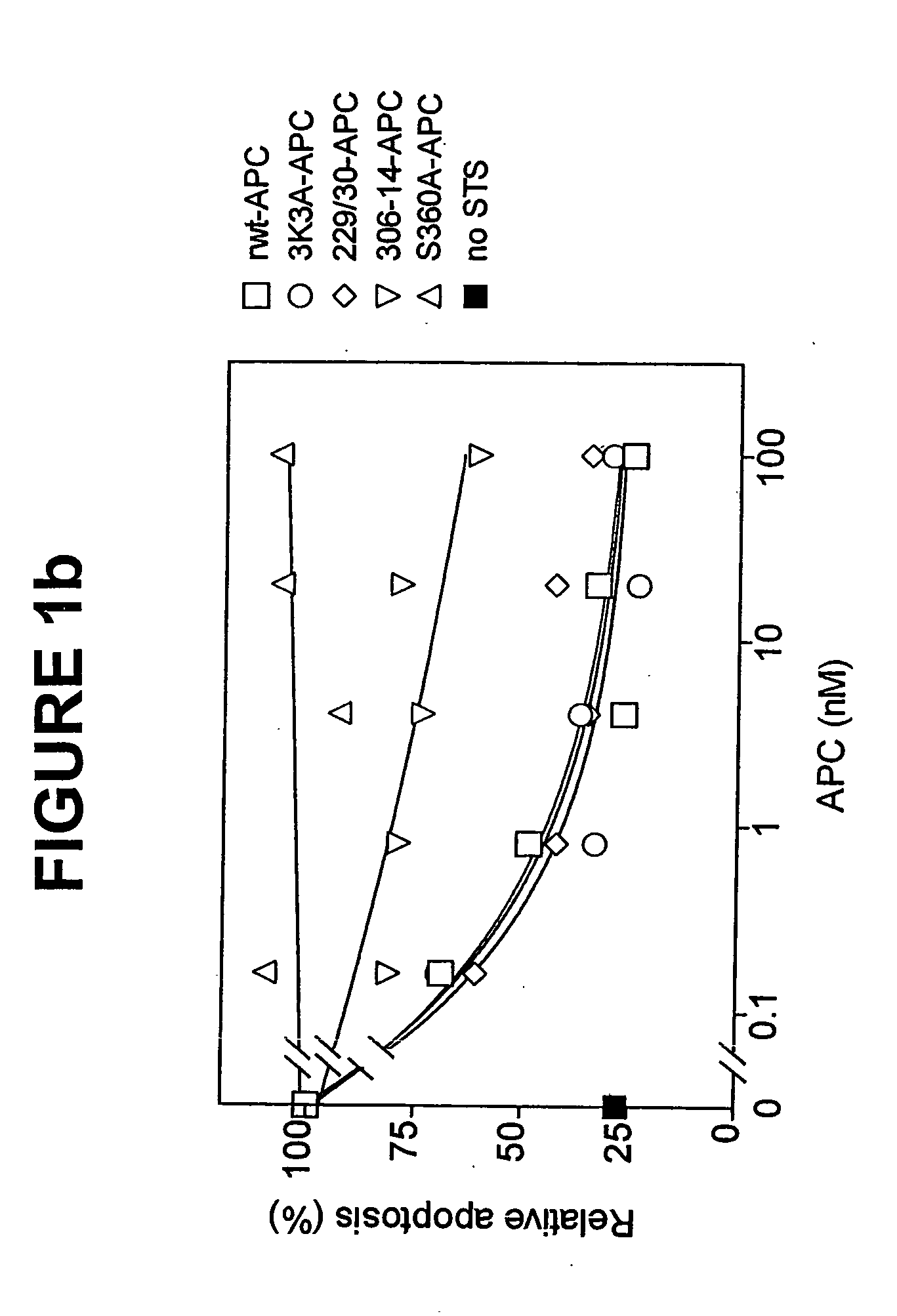
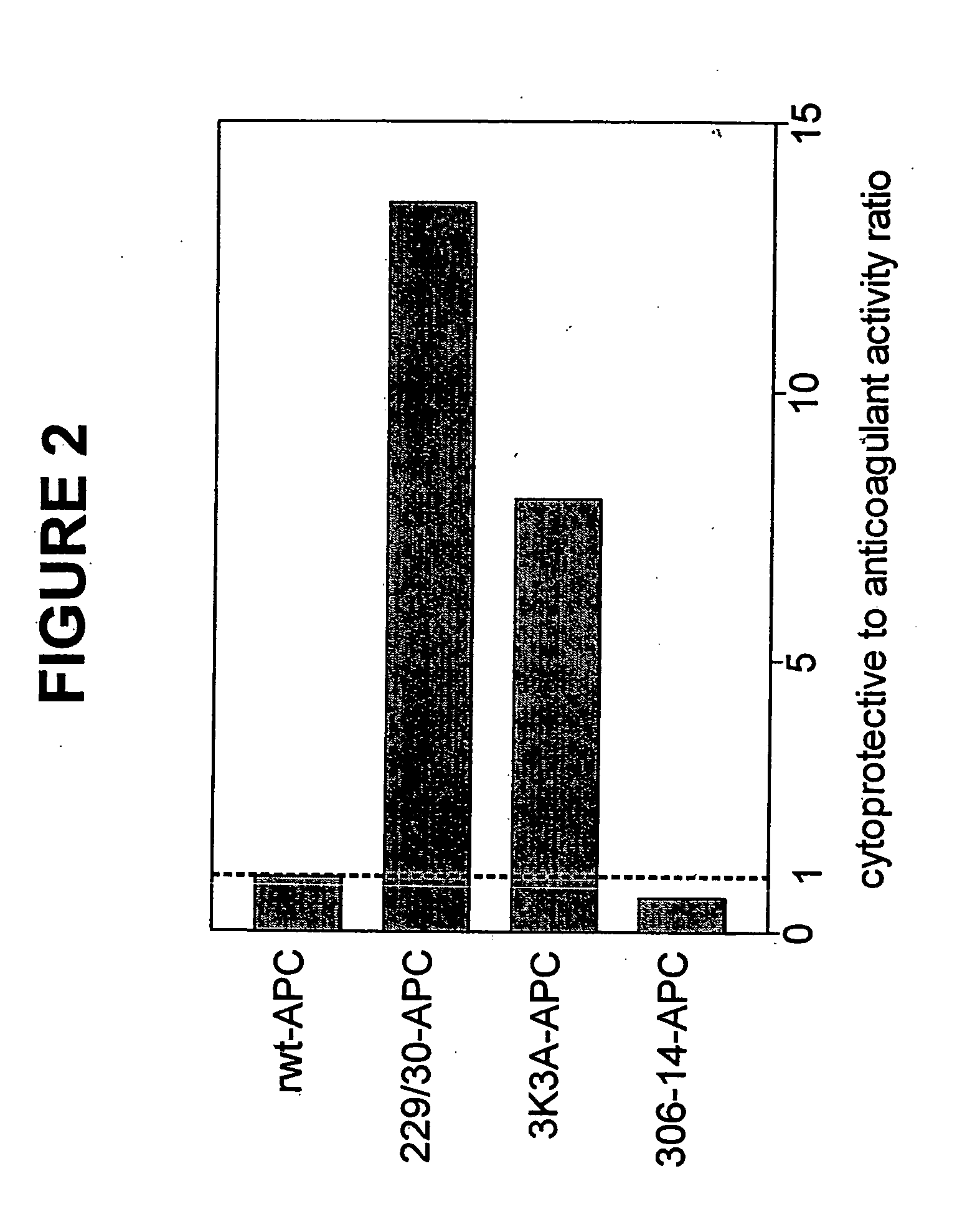
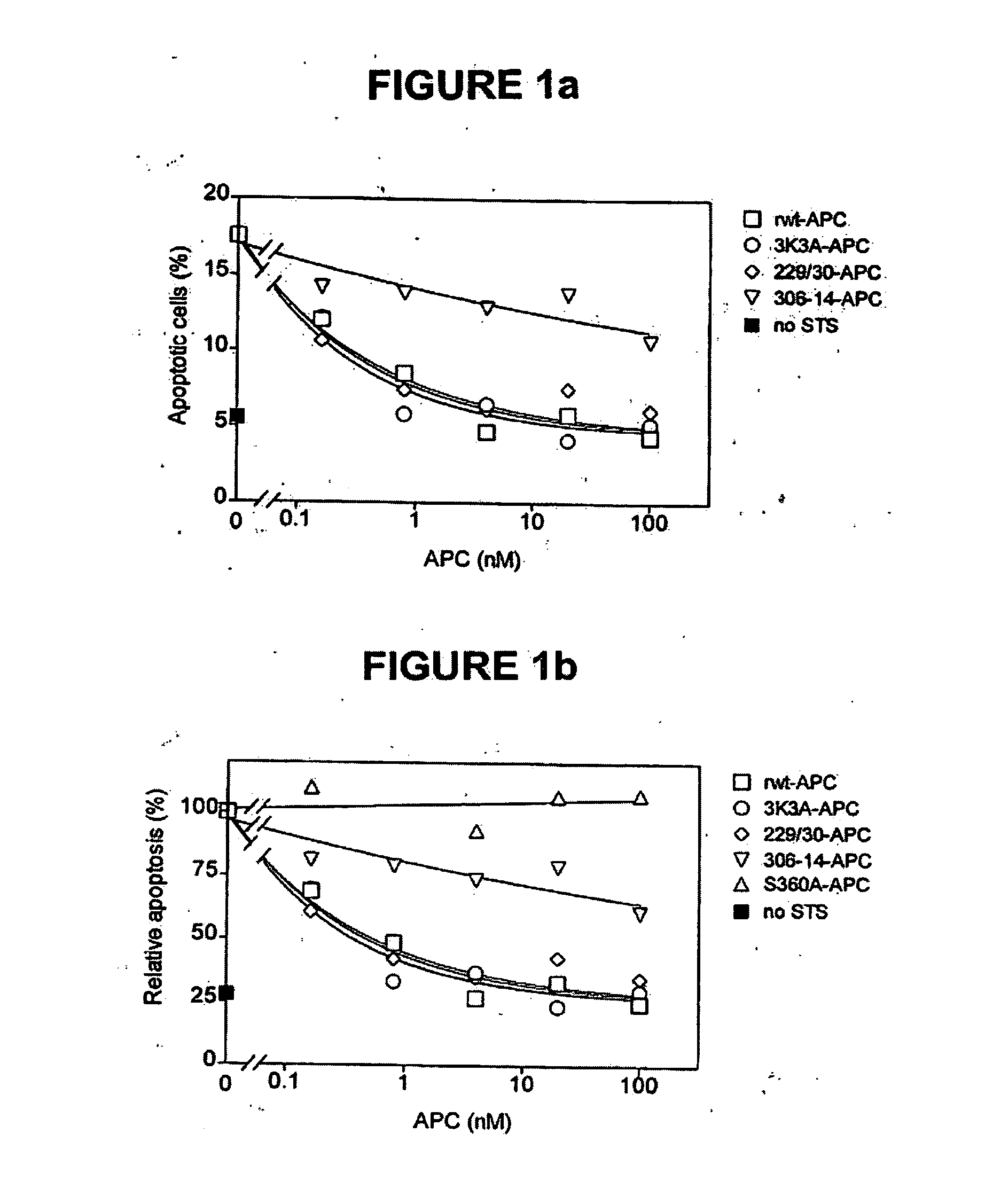
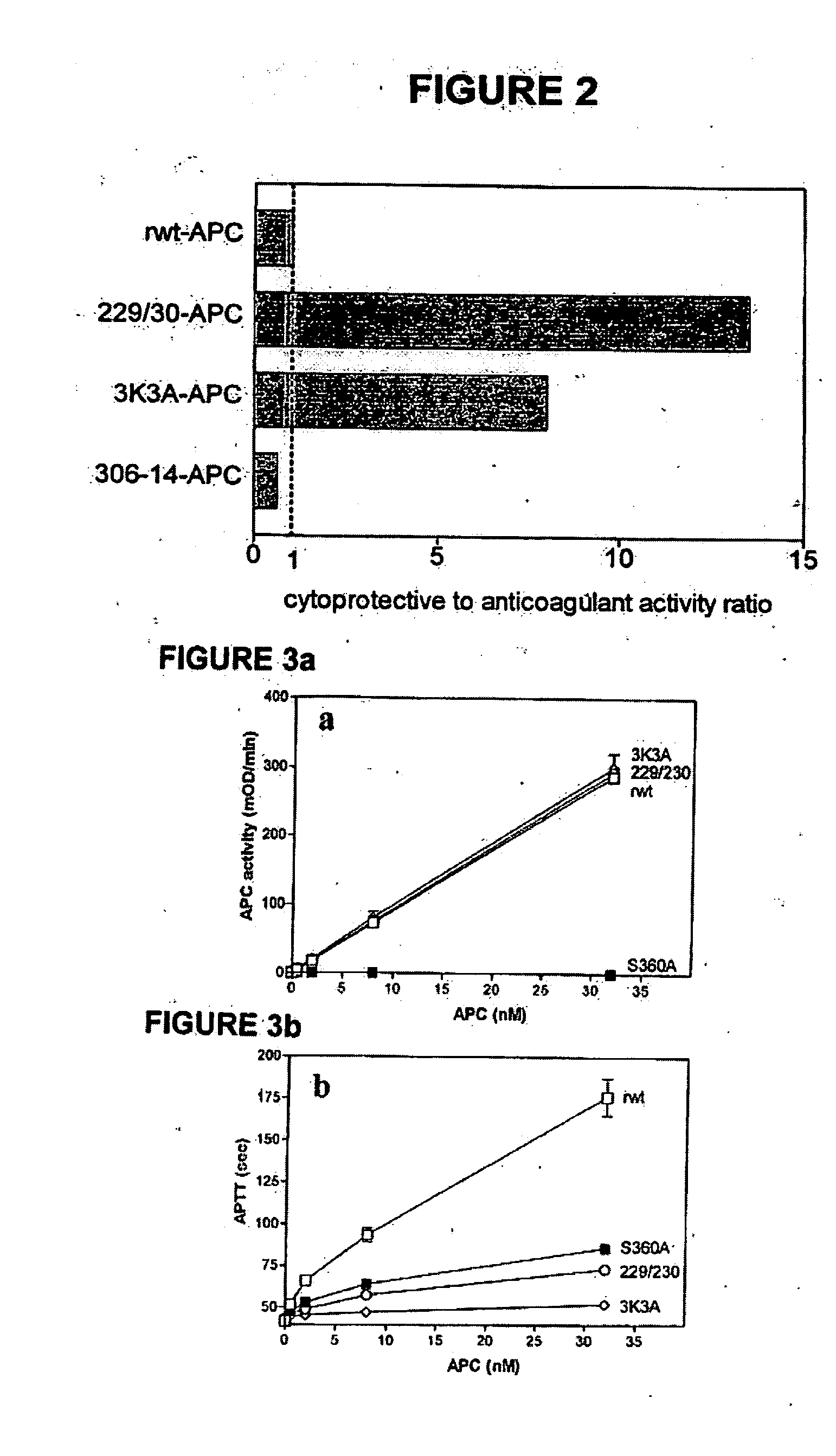
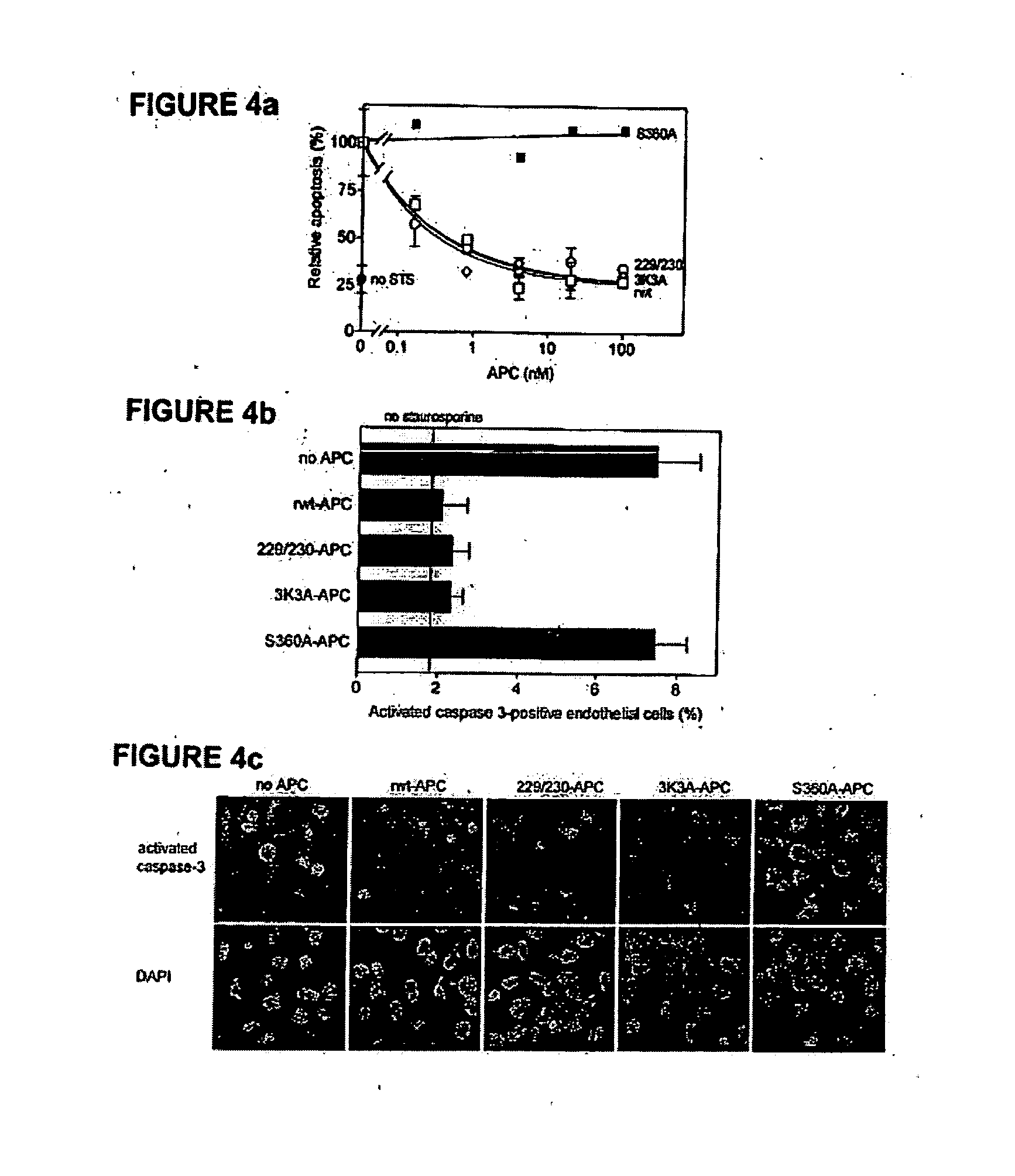
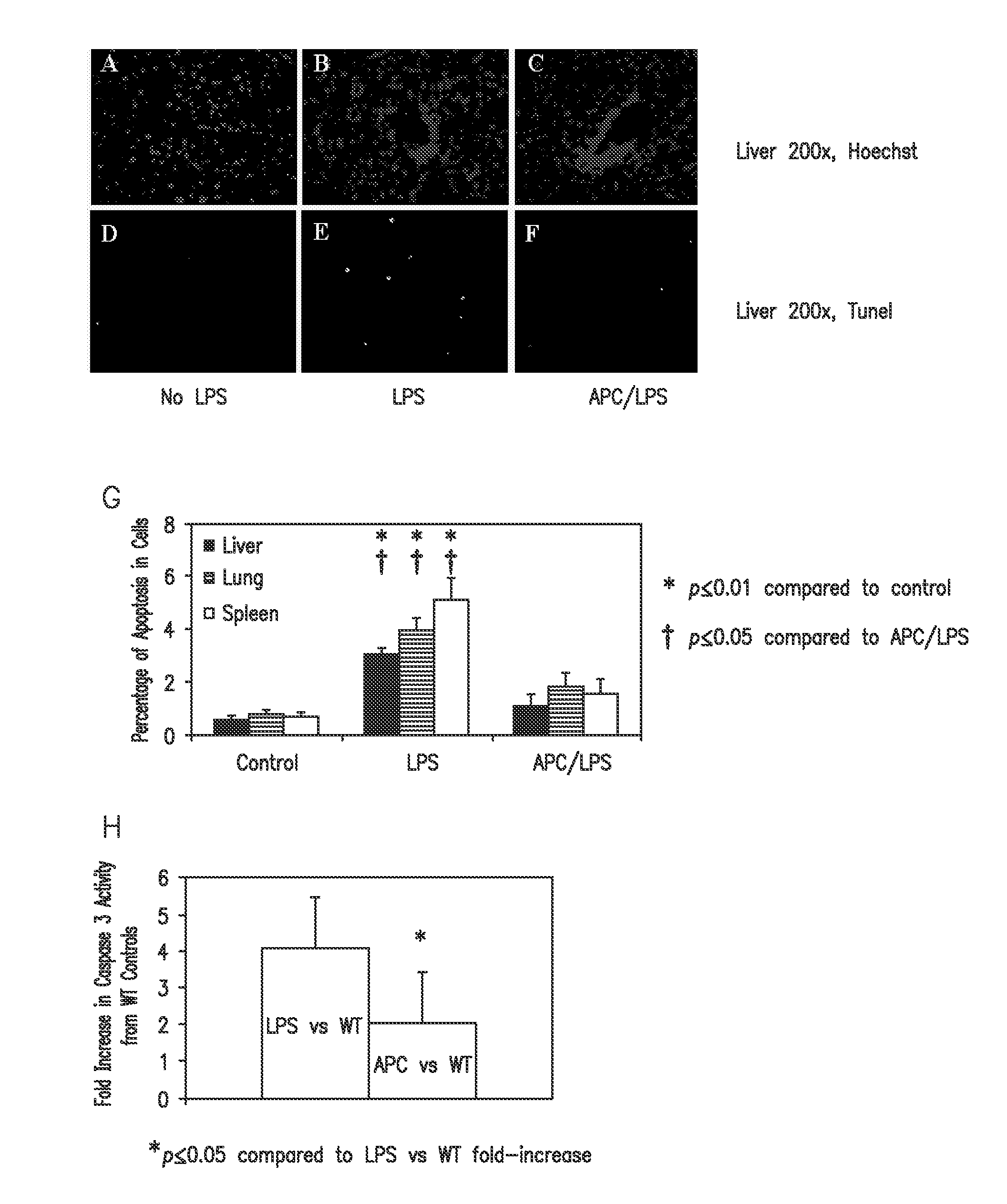
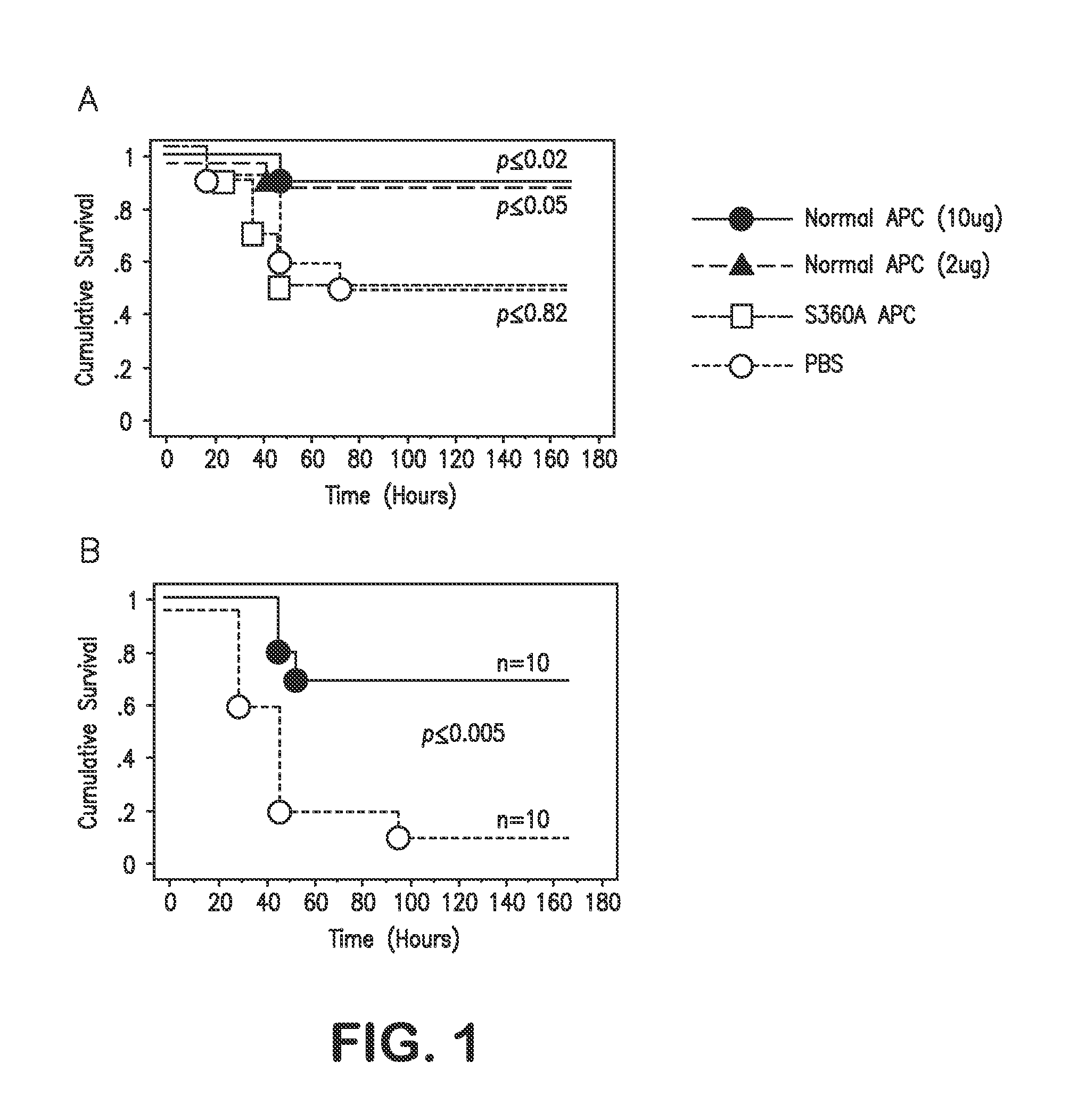
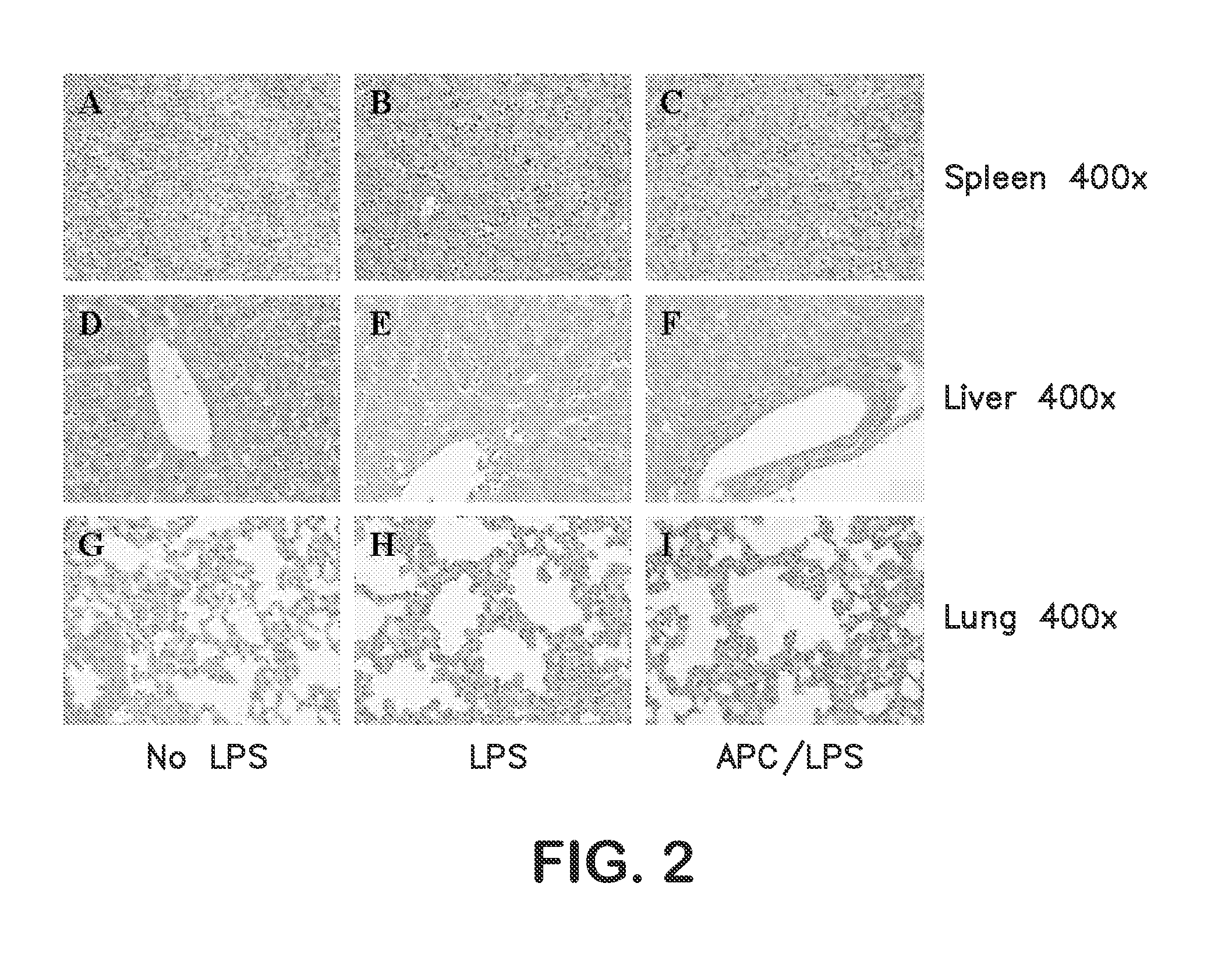
Activated protein C variants with normal cytoprotective activity but reduced anticoagulant activity
ActiveUS7498305B2Reduced activityReduce bleeding riskAntibacterial agentsNervous disorderReperfusion injuryApoptosis
Variants (mutants) of recombinant activated protein C (APC) or recombinant protein C (prodrug, capable of being converted to APC) that have substantial reductions in anticoagulant activity but that retain normal levels of anti-apoptotic activity are provided. Two examples of such recombinant APC mutants are KKK191-193AAA-APC and RR229 / 230M-APC. APC variants and prodrugs of the invention have the desirable property of being cytoprotective (anti-apoptotic effects), while having significantly reduced risk of bleeding. The invention also provides a method of using the APC variants or prodrugs of the invention to treat subjects who will benefit from APC's cytoprotective activities that are independent of APC's anticoagulant activity. These subjects include patients at risk of damage to blood vessels or tissue in various organs caused, at least in part, by apoptosis. At risk patients include, for example, those suffering (severe) sepsis, ischemia / reperfusion injury, ischemic stroke, acute myocardial infarction, acute or chronic neurodegenerative diseases, or those undergoing organ transplantation or chemotherapy, among other conditions. Methods of screening for variants of recombinant protein C or APC that are useful in accordance with the invention are also provided.
Owner:THE SCRIPPS RES INST
Activated protein C variants with normal cytoprotective activity but reduced anticoagulant activity
ActiveUS20050037964A1Reduced anticoagulant activityReduce bleeding riskAntibacterial agentsNervous disorderReperfusion injuryApoptosis
Variants (mutants) of recombinant activated protein C (APC) or recombinant protein C (prodrug, capable of being converted to APC) that have substantial reductions in anticoagulant activity but that retain normal levels of anti-apoptotic activity are provided. Two examples of such recombinant APC mutants are KKK191-193AAA-APC and RR229 / 230M-APC. APC variants and prodrugs of the invention have the desirable property of being cytoprotective (anti-apoptotic effects), while having significantly reduced risk of bleeding. The invention also provides a method of using the APC variants or prodrugs of the invention to treat subjects who will benefit from APC's cytoprotective activities that are independent of APC's anticoagulant activity. These subjects include patients at risk of damage to blood vessels or tissue in various organs caused, at least in part, by apoptosis. At risk patients include, for example, those suffering (severe) sepsis, ischemia / reperfusion injury, ischemic stroke, acute myocardial infarction, acute or chronic neurodegenerative diseases, or those undergoing organ transplantation or chemotherapy, among other conditions. Methods of screening for variants of recombinant protein C or APC that are useful in accordance with the invention are also provided.
Owner:THE SCRIPPS RES INST
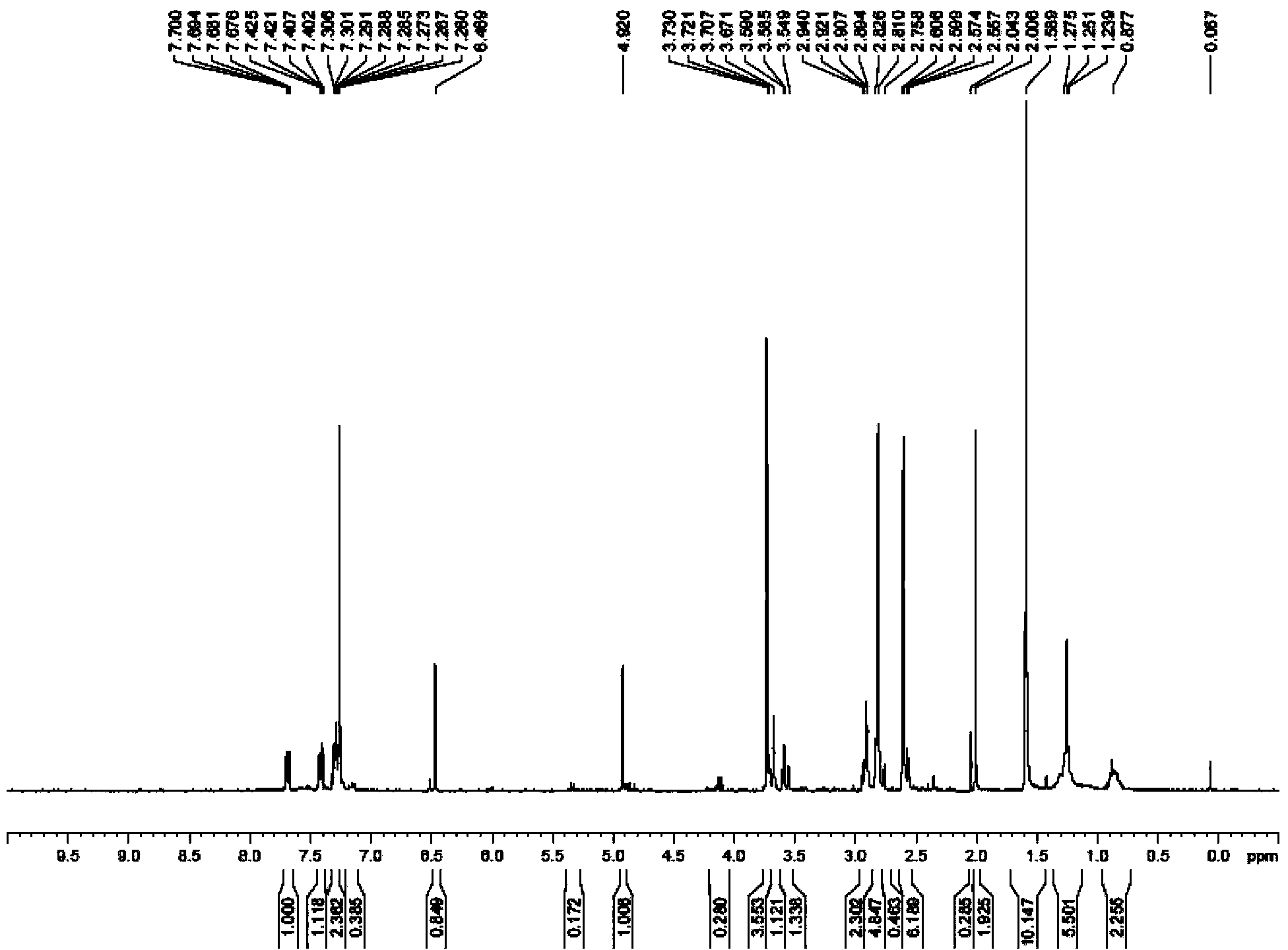
Thienopyridine derivatives, and preparation methods and medical use thereof
ActiveCN103694250AMetabolic avoidanceSolve the problem of resistanceOrganic chemistryBlood disorderClopidogrel resistanceLiver and kidney
The invention belongs to the field of pharmaceutical chemistry technology, and particularly discloses thienopyridine derivatives, and preparation methods and a medical use thereof. Through structural modification of clopidogrel and prasugrel, a series of new thienopyridine derivative compounds are synthesized and mainly include derivatives esterified with ligustrazine formic acid and shikimic acid; the compounds go into a body, then are rapidly metabolized into effective metabolites and ligustrazine formic acid or shikimic acid, successfully keep away from metabolism of CYP2C19 enzyme, can be directly metabolized into active compounds to play a pharmacological function, thereby solving the clopidogrel resistance problem, effectively improving the compound antithrombotic activity, and also having no significant effect on hemorrhage risk; and the compounds have relatively ideal protective function on liver and kidney, and also have potential therapeutic significance for other cardiovascular diseases.
Owner:WUHAN QR PHARMA CO LTD
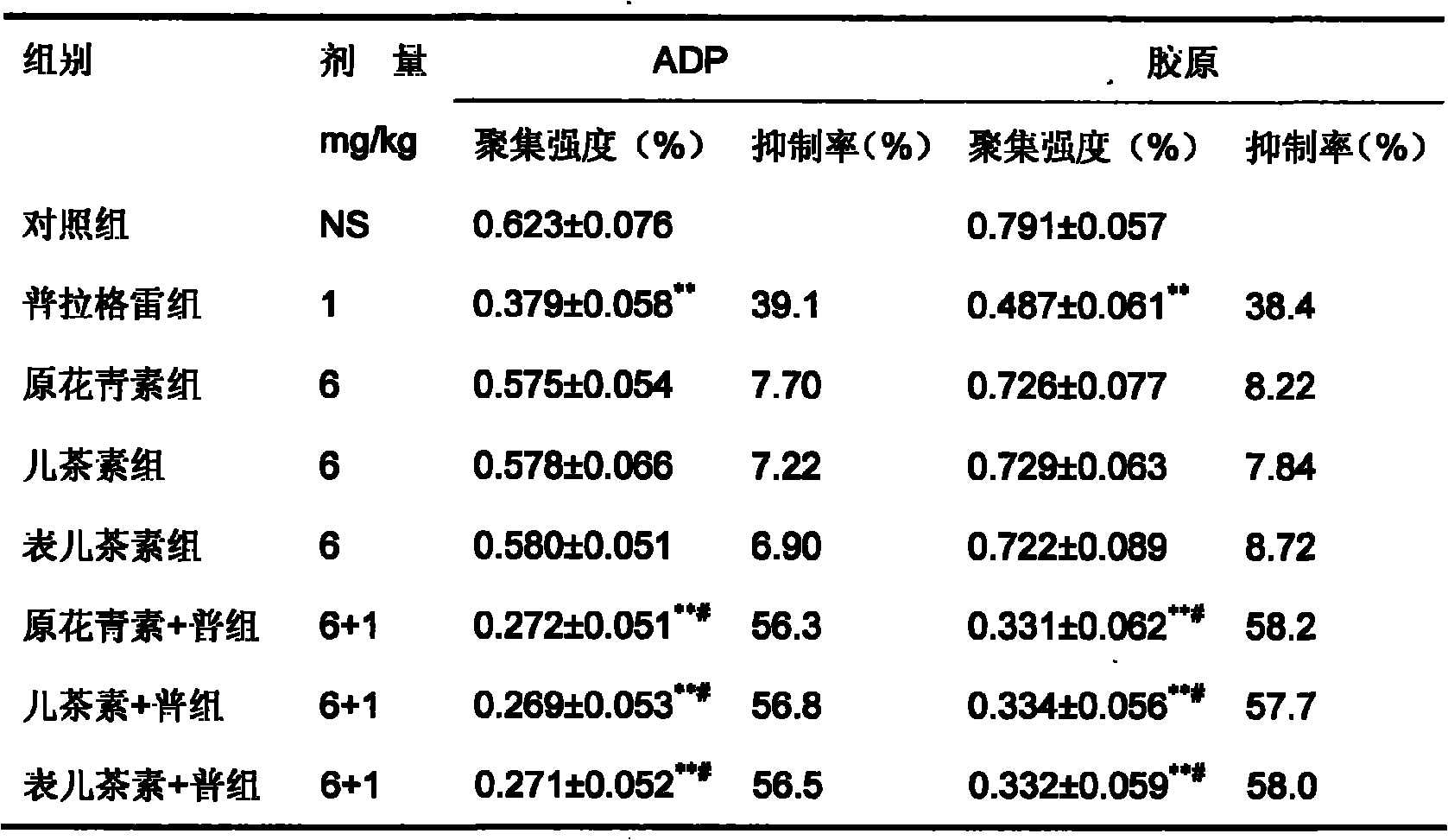
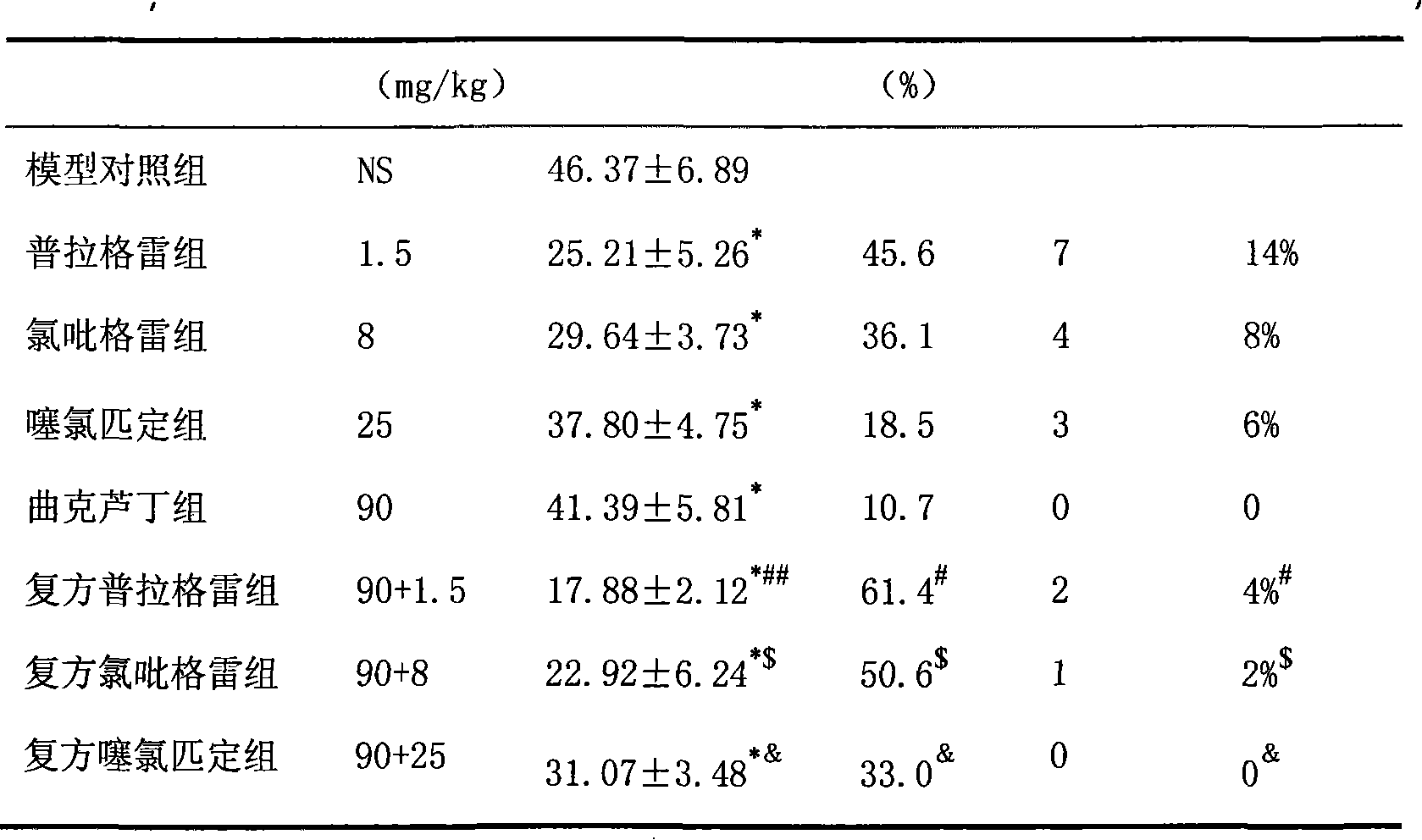
Pharmaceutical composition containing prasugrel
ActiveCN101554378ADid not affect anticoagulant activityHigh anticoagulant activityOrganic active ingredientsBlood disorderThrombusCvd risk
The invention provides a pharmaceutical composition containing active procyanidins and prasugrel or the pharmacologically acceptable salt thereof. The procyanidins and the prasugrel are used in a combined manner, a more effective method for curing thrombotic diseases is found, after trail for many times, the prasugrel and the procyanidins (an extract of traditional Chinese medicine) are creatively and effectively combined together, the effect of applying the procyanidins to inhibit the thrombosis in a combined manner is discovered unexpected in the process of applying the prasugrel to cure the thrombotic diseases, and not only the anticoagulant effect of the prasugrel is free from the influence of the procyanidins, but also a good effect is achieved in the aspect of reducing the adverse reaction of bleeding after the use of the prasugrel and the procyanidins in a combined manner, therefore, the risk of bleeding is greatly reduced when the advantages of good anticoagulant activity and fast effect of the prasugrel during the antiplatelet aggregation are fully exerted, the risk of bleeding of the prasugrel during the antiplatelet aggregation is effectively reduced and the adverse reaction of the prasugrel is greatly reduced.
Owner:LUNAN PHARMA GROUP CORPORATION
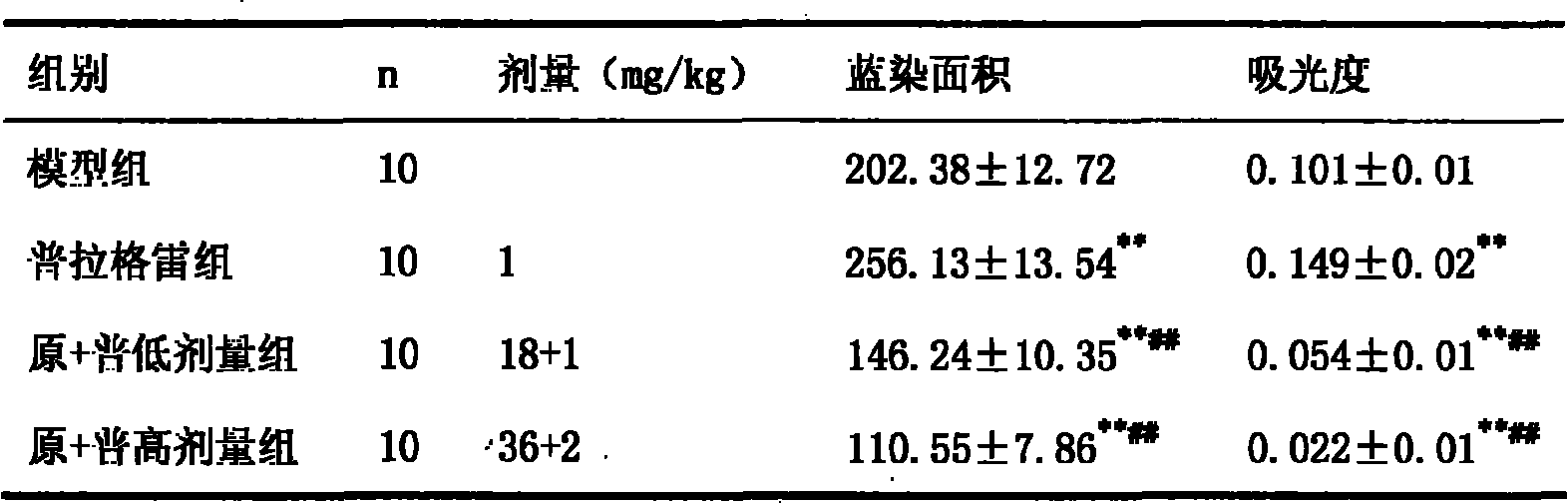
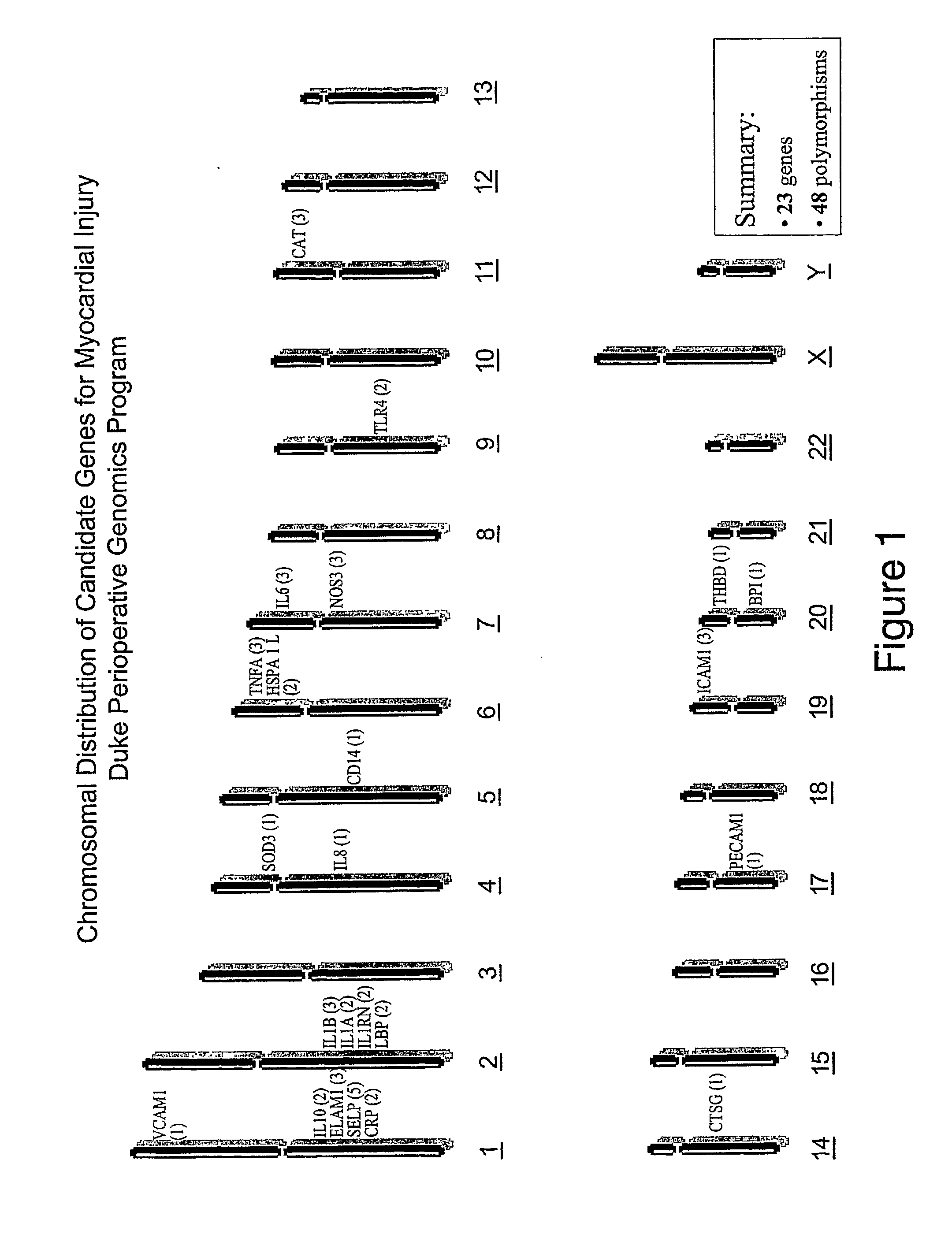
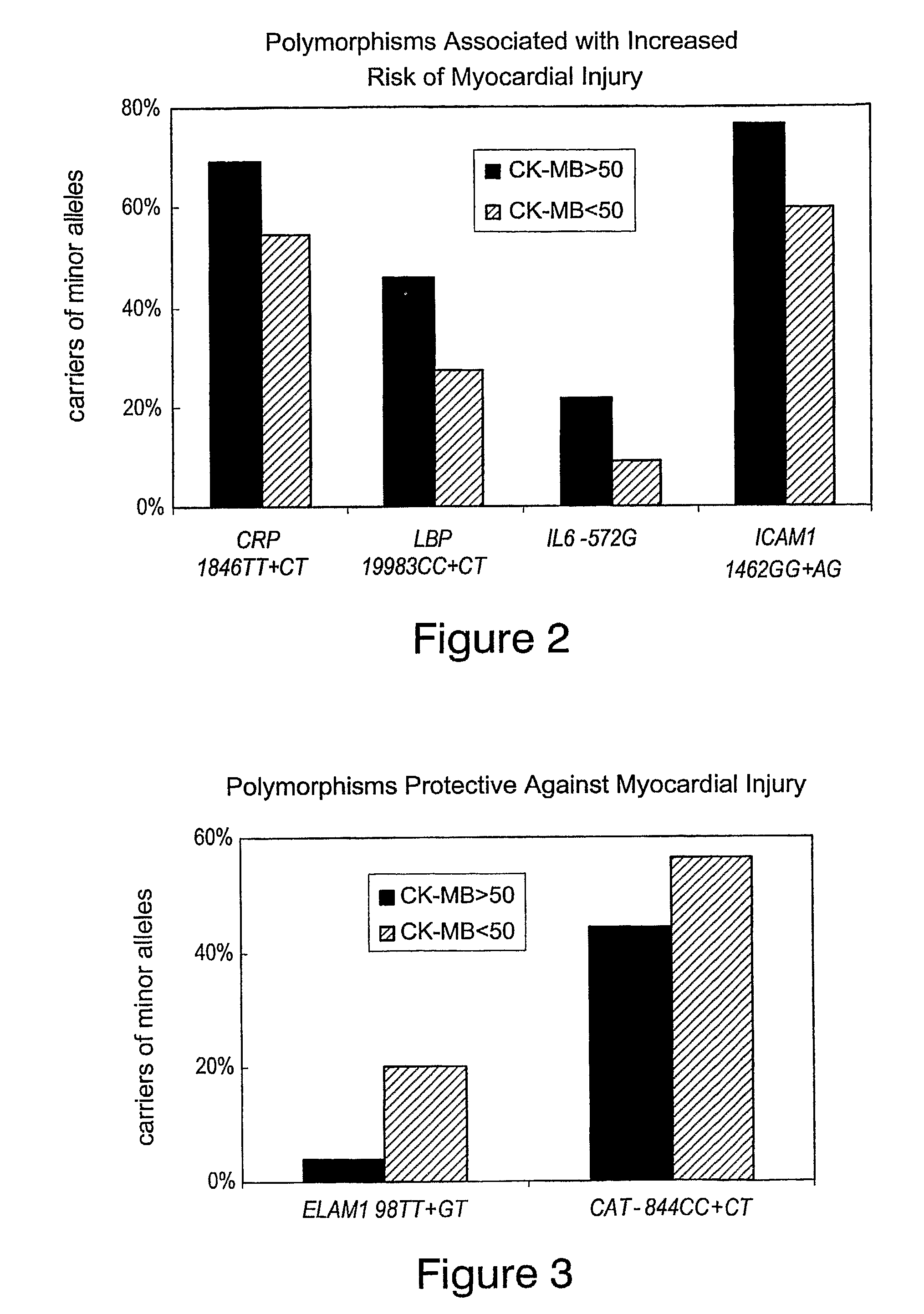
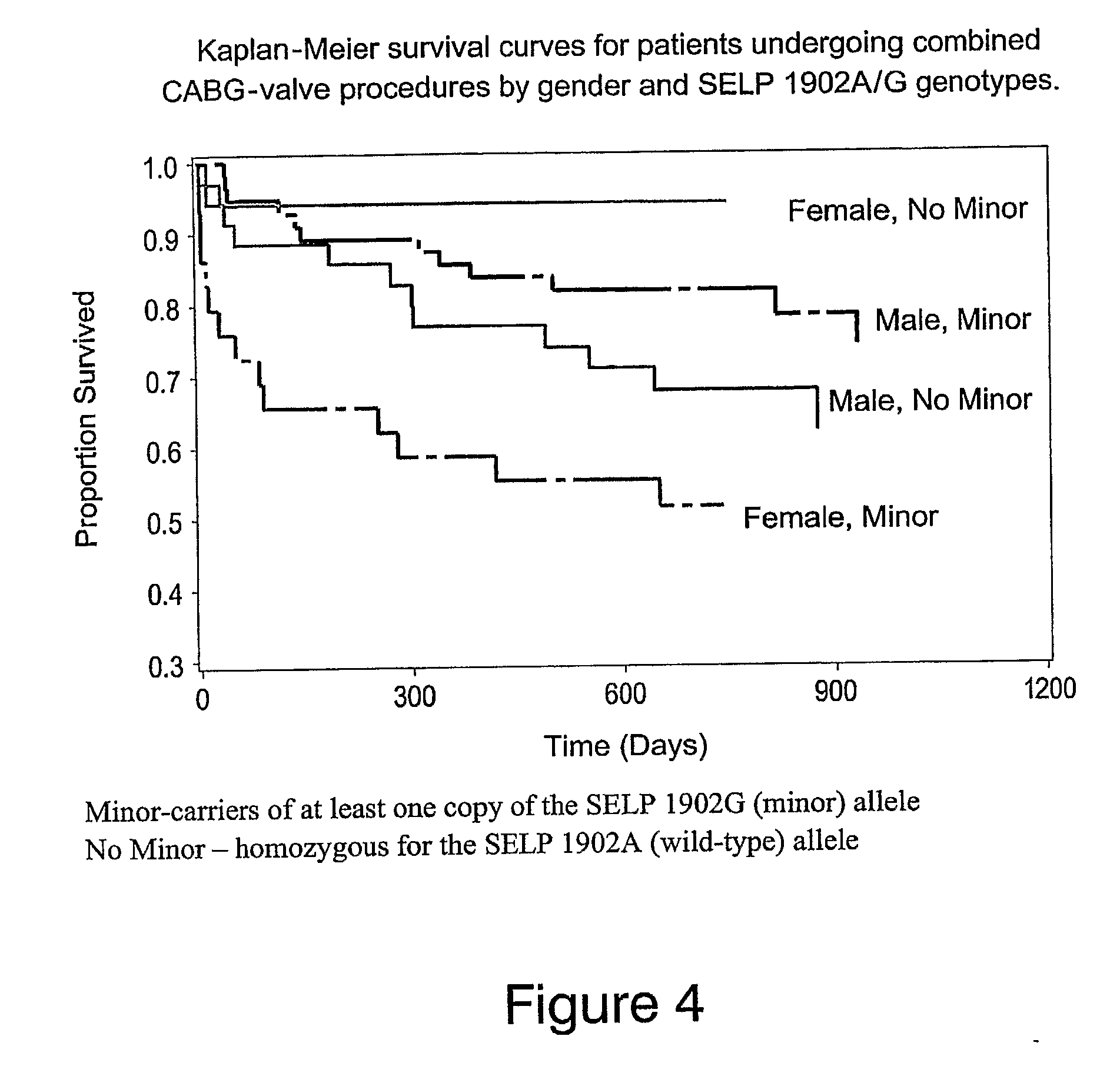
Method of identifying individuals at risk of perioperative myocardial injury, major adverse cardiac events, cognitive decline, arrhythmias, depression or bleeding
The present invention relates to methods of identifying individuals at risk of perioperative myocardial injury, major adverse cardiac events, cognitive decline, arrhythmias, depression and bleeding.
Owner:DUKE UNIV
Activated protein C variants with normal cytoprotective activity but reduced anticoagulant activity
ActiveUS20070042961A1Alleviating and preventing cell damageLess risk of bleedingNervous disorderPeptide/protein ingredientsReperfusion injuryApoptosis
Variants (mutants) of recombinant activated protein C (APC) or recombinant protein C (prodrug, capable of being converted to APC) that have substantial reductions in anticoagulant activity but that retain normal levels of anti-apoptotic activity are provided. Three examples of such recombinant APC mutants are KKK191-193AAA-APC, RR229 / 230AA-APC, and RR229 / 230AA plus KKK191-193AAA-APC. APC variants and prodrugs of the invention have the desirable property of being cytoprotective (anti-apoptotic effects), while having significantly reduced risk of bleeding. The invention also provides a method of using the APC variants or prodrugs of the invention to treat subjects who will benefit from APC's cytoprotective activities that are independent of APC's anticoagulant activity. These subjects include patients at risk of damage to blood vessels or tissue in various organs caused, at least in part, by apoptosis. At risk patients include, for example, those suffering (severe) sepsis, ischemia / reperfusion injury, ischemic stroke, acute myocardial infarction, acute or chronic neurodegenerative diseases, or those undergoing organ transplantation or chemotherapy, among other conditions. Methods of screening for variants of recombinant protein C or APC that are useful in accordance with the invention are also provided.
Owner:THE SCRIPPS RES INST
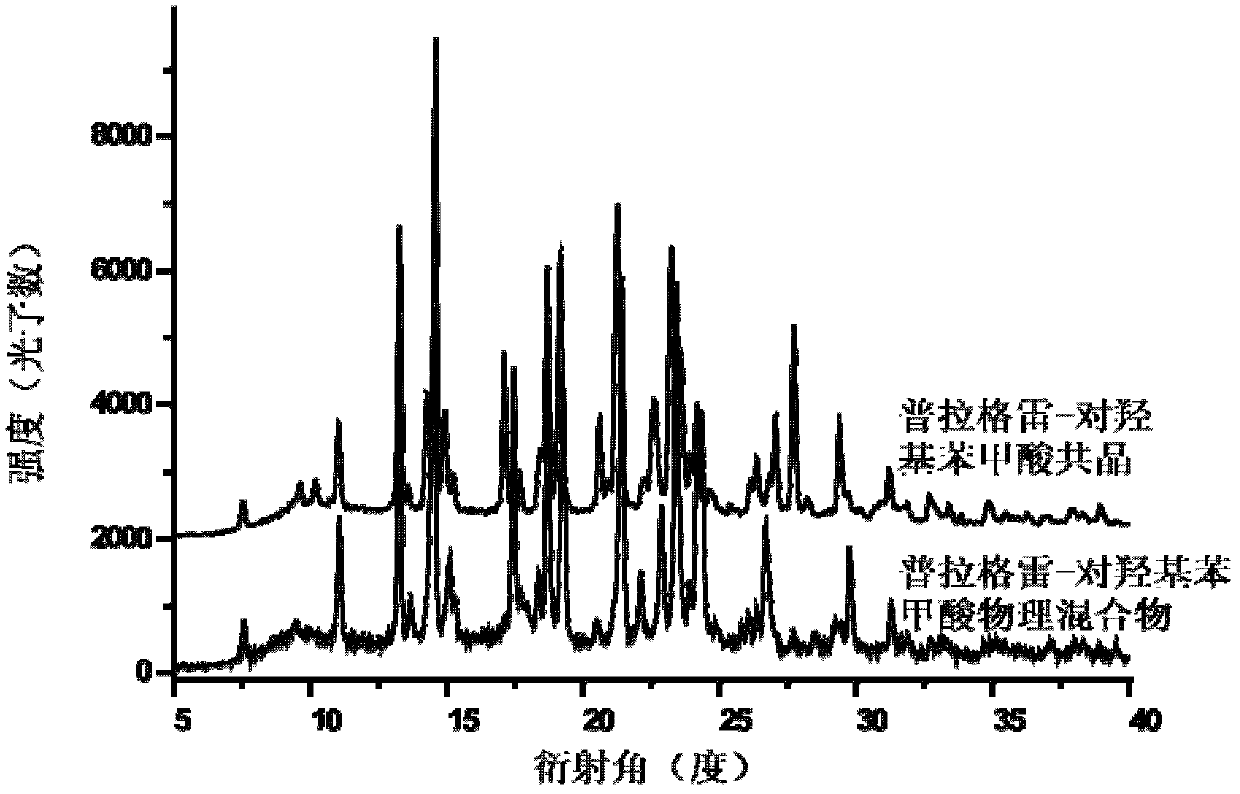
Prasugrel eutectic and preparation method, medicinal composition and application thereof
InactiveCN102617594AReduce humidityReduce dissolution rateOrganic active ingredientsNervous disorderTraditional medicineSolvent
The invention provides a prasugrel eutectic with a structure shown in the specification, and a preparation method, a medicinal composition and application thereof. The prasugrel eutectic does not contain a solvent and is low in hygroscopicity, so that the preparation and storage of Chinese herbal medicines are facilitated. Experiments for testing dissolubility prove that the prasugrel eutectic is relatively low in dissolution speed, so the prasugrel eutectic can be used as a sustained-release agent for prasugrel medicines to overcome the defect of increasing hemorrhagic risks. In addition, the method for preparing the prasugrel eutectic is simple, and easy to operate.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Onset of force development as a marker of thrombin generation
InactiveUS20030199428A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseCoronary artery disease
Platelet contractile force (PCF) is used as a surrogate marker of thrombin generation. PCF generation occurs concomitant with the burst of prothrombin fragment F 1+2 release. The time between assay start and PCF onset is identified as the thrombin generation time (TGT), and is used in assessing risk of bleeding, in diagnosing various disorders, and in monitoring the effects of pharmaceutical and other treatments. TGT is prolonged in clotting factor deficiencies and in the presence of direct and indirect thrombin inhibitors. TGT shortens to normal with clotting factor replacement and shortens with administration of rVIIa. TGT is short in thrombophilic states such as coronary artery disease, diabetes and thromboangiitis obliterans and prolongs toward normal with oral and intravenous anticoagulants.
Owner:HEMODYNE
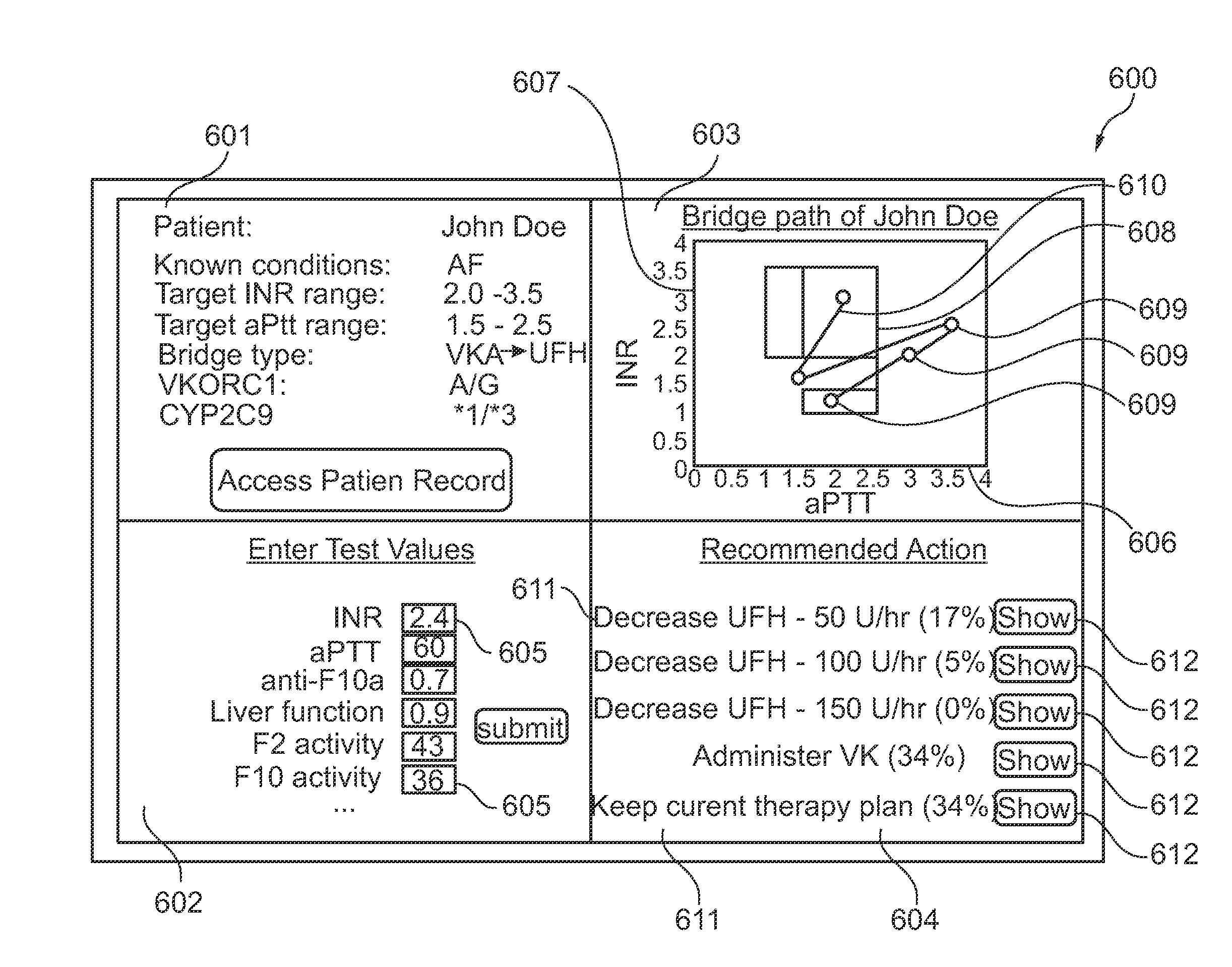
Method of visualizing a bridge therapy process
ActiveUS20140118356A1Good bridgingBalance securityMedical simulationDrawing from basic elementsThrombusPharmacokinetic modeling
The present invention provides for a simultaneous graphical representation, a risk of bleeding and a risk of thrombosis providing a visualized bridge therapy process. Furthermore, the present invention provides for a computer-based prediction of the haemostatic situation of the examined blood circulation by using a combination of a biochemical model and a pharmacokinetic model for calculation or another mathematical representation of the blood circulation.
Owner:KONINKLJIJKE PHILIPS NV
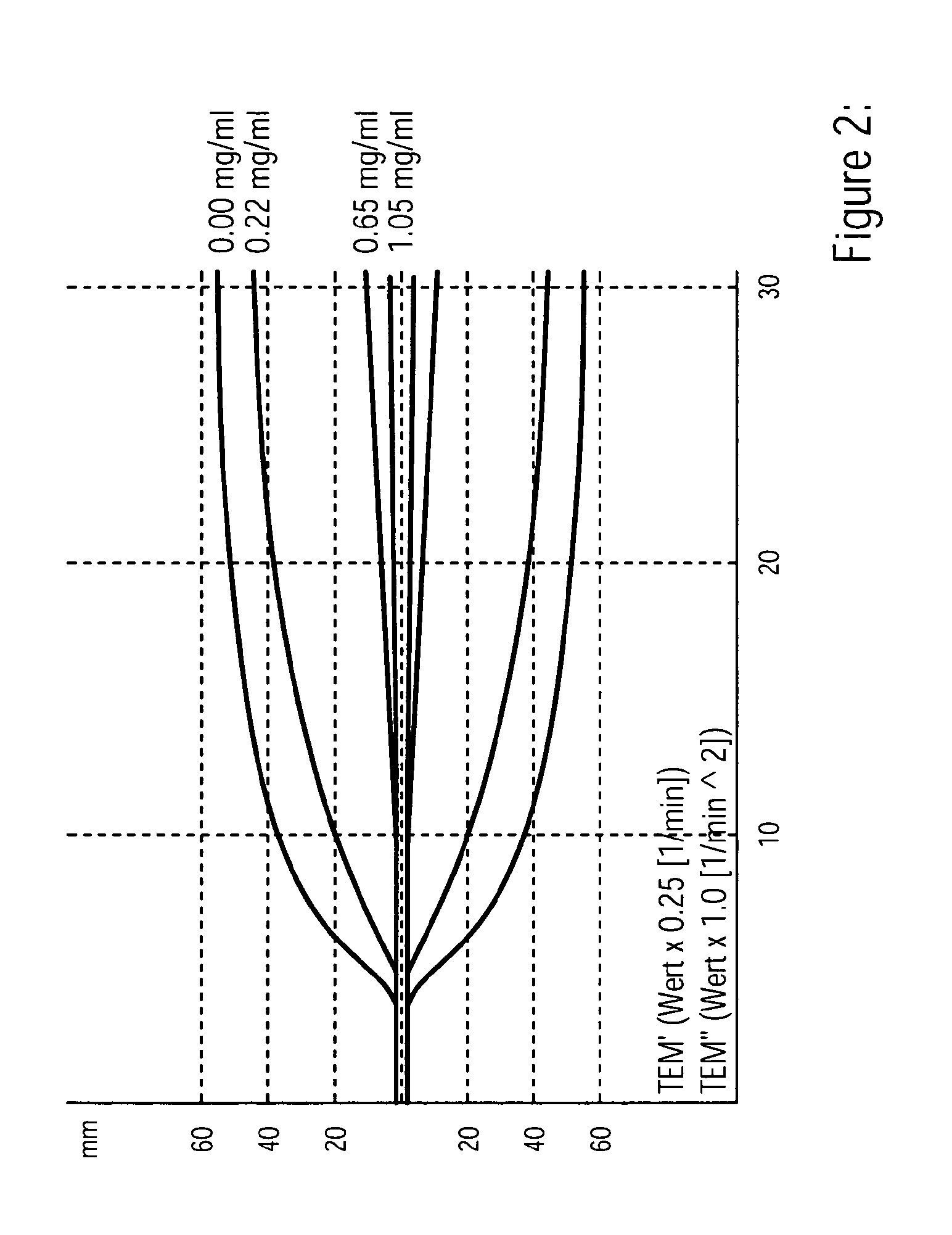
Diagnostic in vitro method for assessing von willebrand disease and increased bleeding risk associated with von willebrand disease and acquired or congenital disorders of platelet function
InactiveUS20100273206A1Superior in predicting bleeding riskMicrobiological testing/measurementDisease diagnosisPoint of careFactor ii
The invention relates to an in-vitro method for diagnosing Von Willebrand Disease (VWD) and an increased bleeding risk associated with Von Willebrand Disease and / or acquired or congenital platelet function defects that reduce the interactions of Von Willebrand Factor (VWF) with platelets. The in-vitro method of the invention may also be used to diagnose further bleeding risks. The test is suitable for use as a screening test based on whole blood and has the additional benefit of being suitable as a point of care test. The method involves the incubation of a sample containing platelets and hemostasis factors with an activator of platelet aggregation and the measurement of the viscoelastic change after inducing coagulation, e.g., by means of thromboelastography (TEG).
Owner:CSL BEHRING GMBH
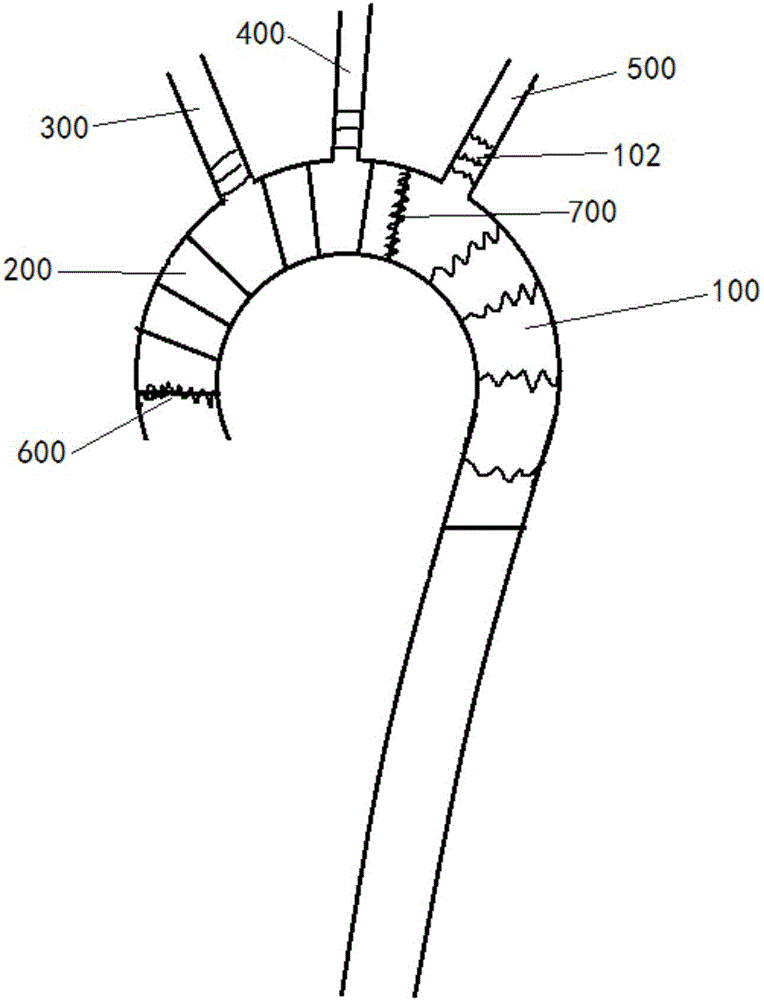
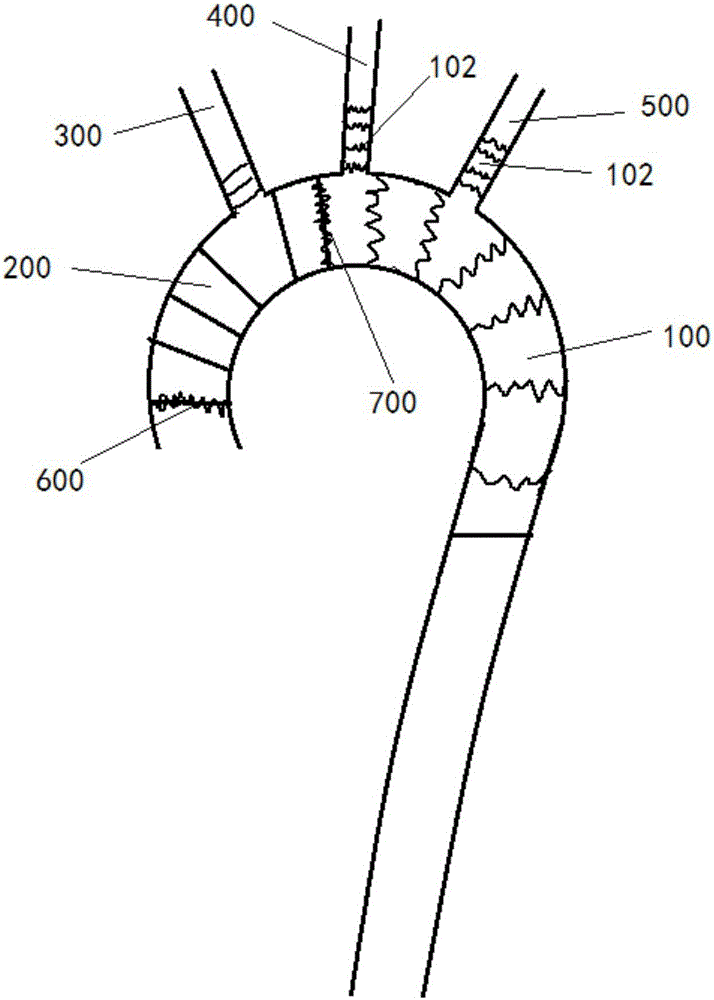
Blood vessel prosthesis stent for Stanford type A aortic dissection
InactiveCN106308975AReduce the number of vascular anastomosisImprove the effect of surgical treatmentStentsBlood vesselsVascular anastomosisCirculatory arrest time
The invention discloses a blood vessel prosthesis stent for Stanford type A aortic dissection. The blood vessel prosthesis stent comprises a main stent body, wherein a single branch or two branches is or are fixed at a proximal end of the main stent body. With the adoption of the blood vessel prosthesis stent, the amount of vascular anastomosis of a patient with Stanford type A aortic dissection affecting the aortic arch part can be effectively reduced, the surgical procedures are simplified, the risk of hemorrhage is reduced, deep hypothermic circulatory arrest and low-flow cerebral protection time in the period of cardiovascular arrest are shortened, so that the surgical treatment effect of the patient with the Stanford type A aortic dissection is improved, and the blood vessel prosthesis stent can be popularized in primary hospitals.
Owner:HARBIN MEDICAL UNIVERSITY
Application of lauromacrogol injection as drug for curing cesarean scar pregnancy
InactiveCN105412009APromote formationReduce bleeding riskOrganic active ingredientsPharmaceutical delivery mechanismInjection siteFibrosis
The invention relates to the field of lauromacrogol injection drug application, and discloses application of lauromacrogol injection as a drug for curing cesarean scar pregnancy. The drug is applied according to the following steps: step (1), a focus scope, a muscular layer thickness and the blood supply situation of a focus location are determined by ultrasound contrast; step (2), the vagina is conventionally disinfected and a towel is spread at a lithotomy position of a patient; step (3), under ultrasonic guidance, the lauromacrogol injection is injected at a peripheral muscular layer of a gestational sac and around the gestational sac, until annular or flaky reinforcement of the gestational sac is seen under ultrasound and peripheral blood is sparse, wherein the specification of the lauromacrogol injection is that each 10ml of injection contains 100mg of lauromacrogol. According to the application of the lauromacrogol injection as the drug for curing cesarean scar pregnancy, the lauromacrogol injection is adhered to the inside of a vessel at the injection site, thus prompting a fibrosis strip to replace the pathological vessel, resulting in permanent occlusion of the vessel around the gestational sac, interdicting an open vessel at the pathological location, and greatly reducing bleeding risk in induced abortion operation.
Owner:张淑珍
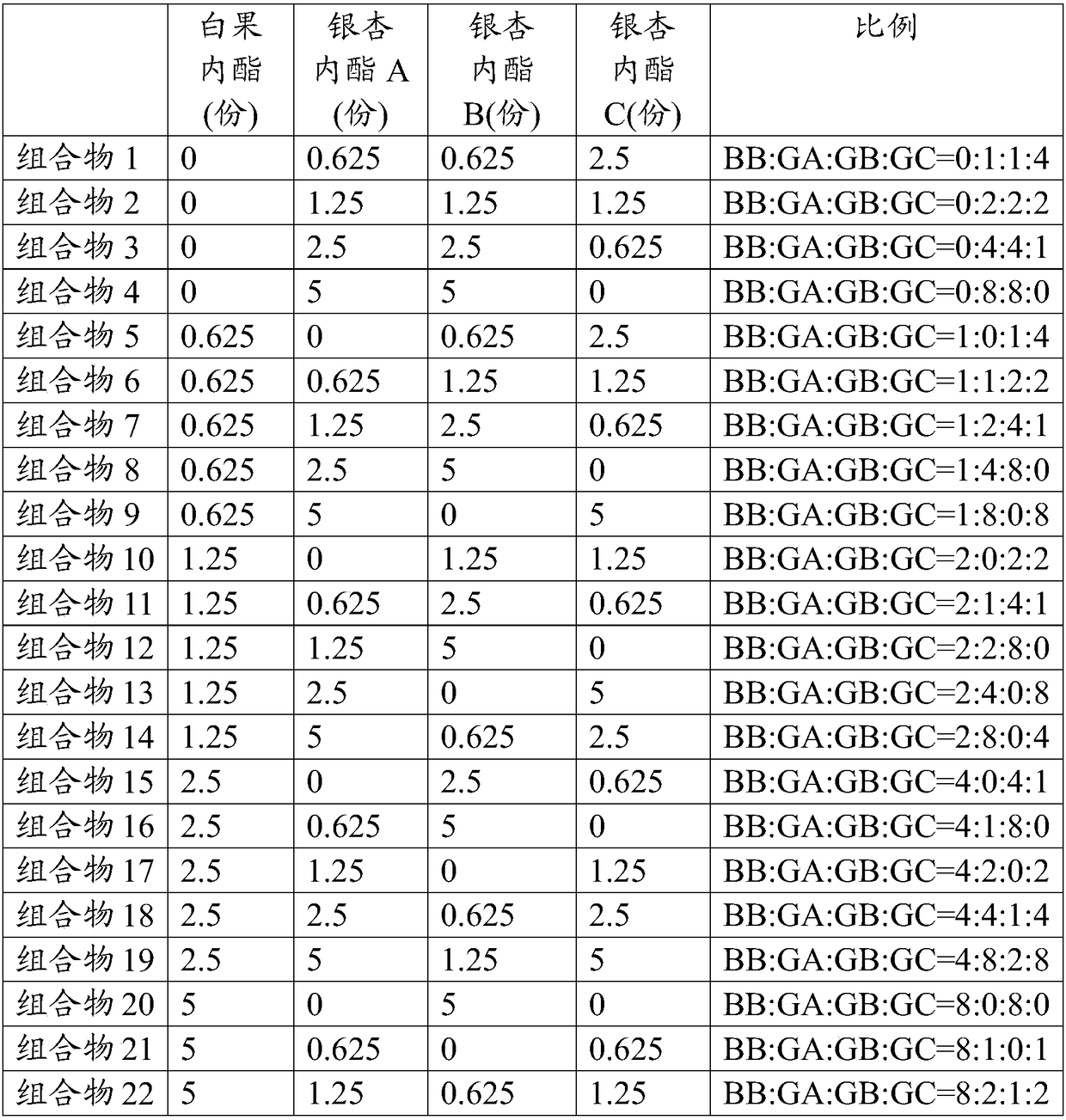
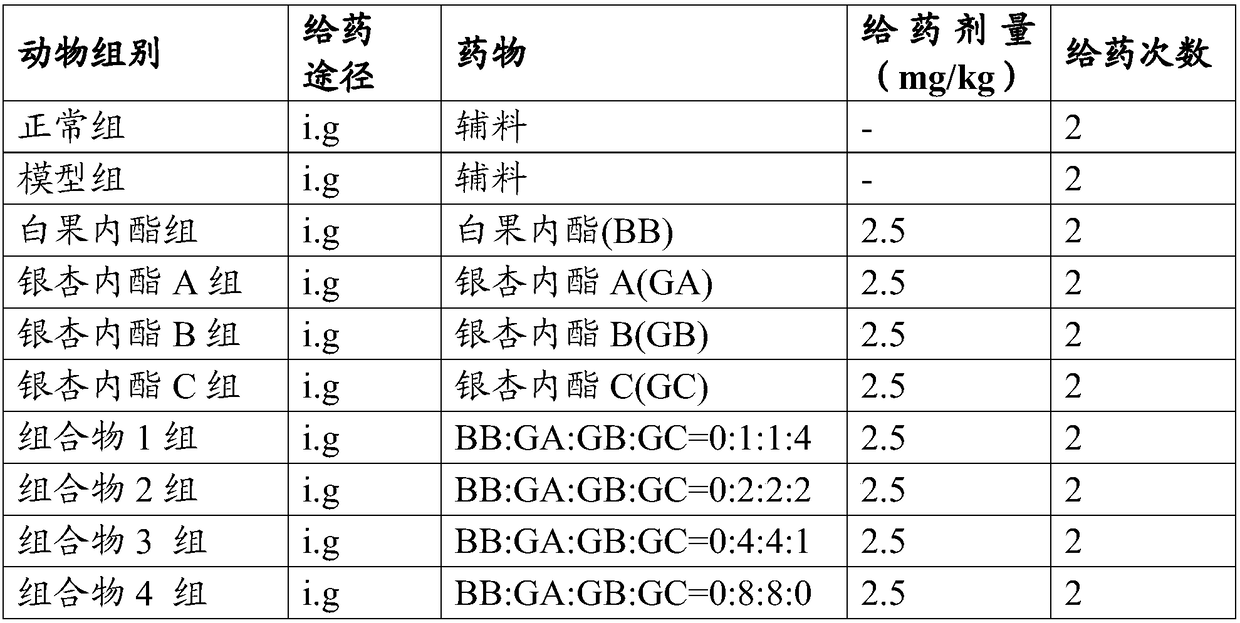
Composition containing ginkgo terpene lactones and medical application thereof
ActiveCN108926556AImprove blood compositionReduce bleeding riskOrganic active ingredientsBlood disorderBlood componentAdditive ingredient
The present invention relates to an application of a composition containing ginkgo terpene lactones in preparation of a medicament for preventing and / or treating venous thromboembolic diseases. The invention also relates to a composition containing the ginkgo terpene lactones and xaban family medicaments, and an application thereof in preparation of the medicament for preventing and / or treating the venous thromboembolic diseases. The composition containing the ginkgo terpene lactones and the composition containing the ginkgo terpene lactones and the xaban family medicaments are effective against formation of the venous thrombus (anticoagulation and improving blood composition) and treat related diseases involving venous thromboembolism, so that an application range of a compound of the ginkgo terpene lactones expands, and a new option, which has the characteristics of lower bleeding risk, suitable price, definite curative effect, safety and reliability, and convenient usage, is provided for treatment of venous thrombus.
Owner:CHENGDU BAIYU PHARMA CO LTD
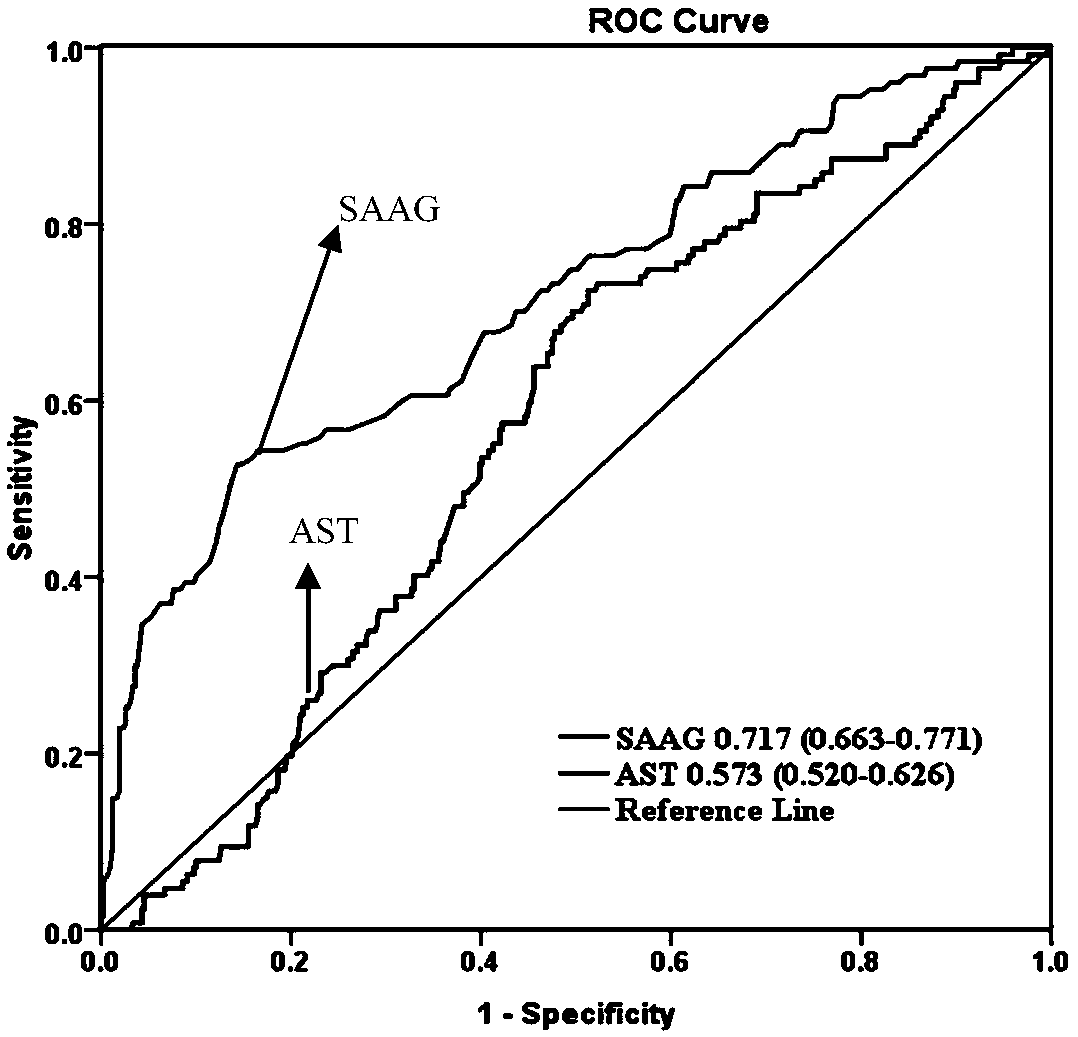
Method and system for evaluating bleeding risk of esophagus and stomach fundus varicosity of cirrhosis patient
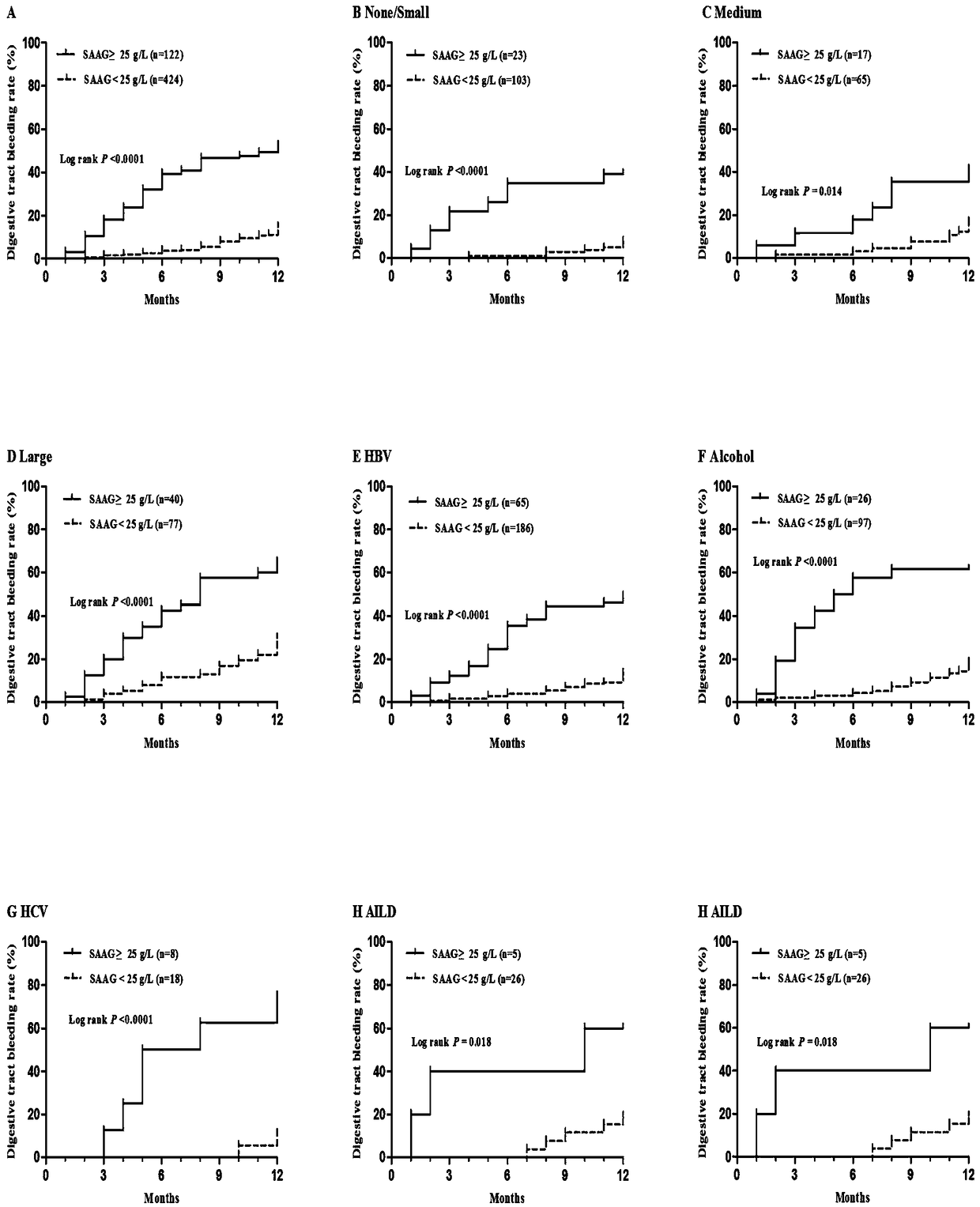
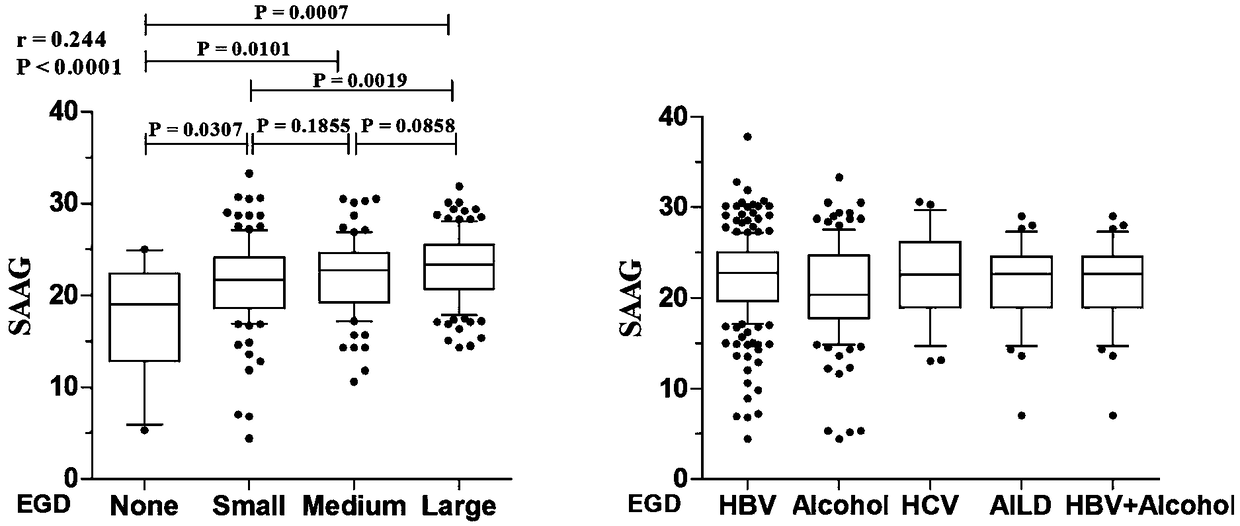
The invention belongs to the technical field of medicine and particularly relates to a method and system for evaluating the bleeding risk of esophagus and stomach fundus varicosity of a cirrhosis patient. It is discovered through research that the SAAG and varix degree of the esophagus and stomach fundus of the patient with cirrhosis ascites have obvious relevance, and the higher the level of therelevance is, the more serious the stomach fundus varicosity of the esophagus is; when SAAG is larger than or equal to 25 g / L, the bleeding risk of esophagus and stomach fundus phleborrhexis of the patient within one year is obviously increased. By means of the method and system, the value of predicting bleeding of varicosity and phleborrhexis of the esophagus and stomach fundus within one year bythe system can be evaluated.
Owner:BEIJING DITAN HOSPITAL CAPITAL MEDICAL UNIV
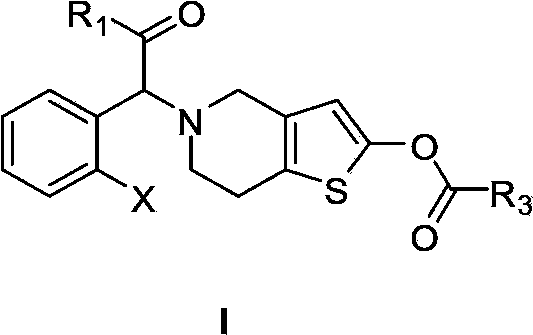
Tetrahydrothienopyridine deuterated derivative as well as preparation method and pharmaceutical application thereof
ActiveCN114736215AImprove conversion rateSolve the problem of resistanceOrganic active ingredientsOrganic chemistry methodsDiseaseThienopyridine Derivatives
The invention discloses a tetrahydrothienopyridine deuterated derivative as well as a preparation method and pharmaceutical application thereof. The derivative can be used for preparing medicines for preventing or treating thrombus-related diseases. The thienopyridine derivative can balance and optimize the safety and effectiveness of a medicine, the thienopyridine derivative is prevented from being hydrolyzed too fast in the gastrointestinal tract, and a better treatment effect is achieved on the premise that the bleeding risk of a patient is not increased.
Owner:ZHONGRONG KAITE BEIJING BIOTECH CO LTD
Method for establishing bleeding risk predicting model of acute coronary syndrome after interventional therapy
PendingCN110364261AEasy to observeReduce the incidence of bleedingMedical simulationHealth-index calculationMedical recordDecision taking
Owner:上海派兰数据科技有限公司
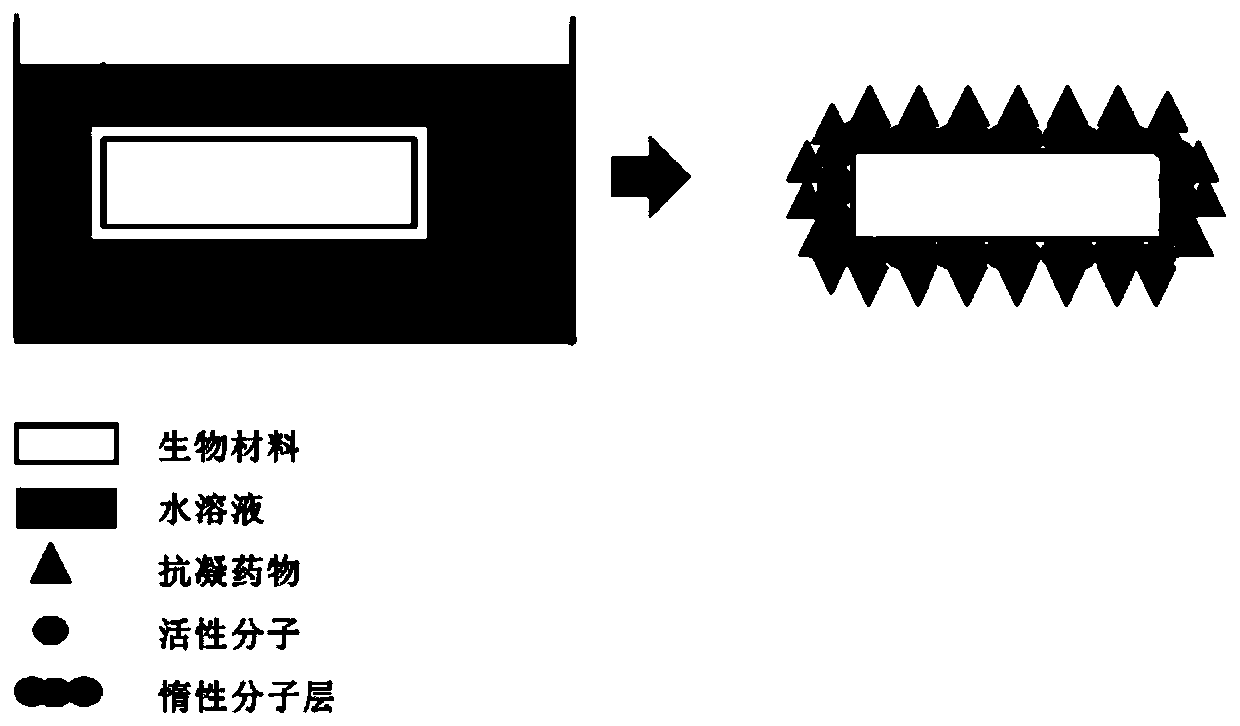
Method for preparing efficient blood coagulation resistant biomaterial with aqueous solution and corresponding material
ActiveCN110755697AGood anticoagulant effectOntology performance impactSurgeryPharmaceutical containersDialysis membranesCardiovascular stent
The invention discloses a method for preparing an efficient blood coagulation resistant biomaterial with an aqueous solution. The aqueous solution has a self-assembly characteristic, is high in biochemistry activity, can form hydrogen bonds with anticoagulation / antithrombus medicines, and has the characteristic of being firmly attached to the biomaterial, so that the surface of the biomaterial (such as a blood purification film, a blood purification pipeline, a cardiovascular stent and an artificial blood vessel) has anticoagulation / antithrombus activity. The surface modification process disclosed by the invention is simple in experimental condition, high in controllability, economical and environment-friendly; and the aqueous solution is high in shaping properties, so that the aqueous solution is basically suitable for anticoagulation / antithrombus modification of the surface of the biomaterial in any shape. The dopamine hydrochloride aqueous solution is prepared, a micromolecule anticoagulant medicine-argatroban is grafted to the surface of a haemodialysis film, the haemodialysis film having anticoagulation / antithrombus activity is successfully prepared, the usage of a whole bodyanticoagulant is reduced or avoided, and the bleeding risk of a haemodialysis patient is reduced.
Owner:HUNAN PROVINCIAL PEOPLES HOSPITAL
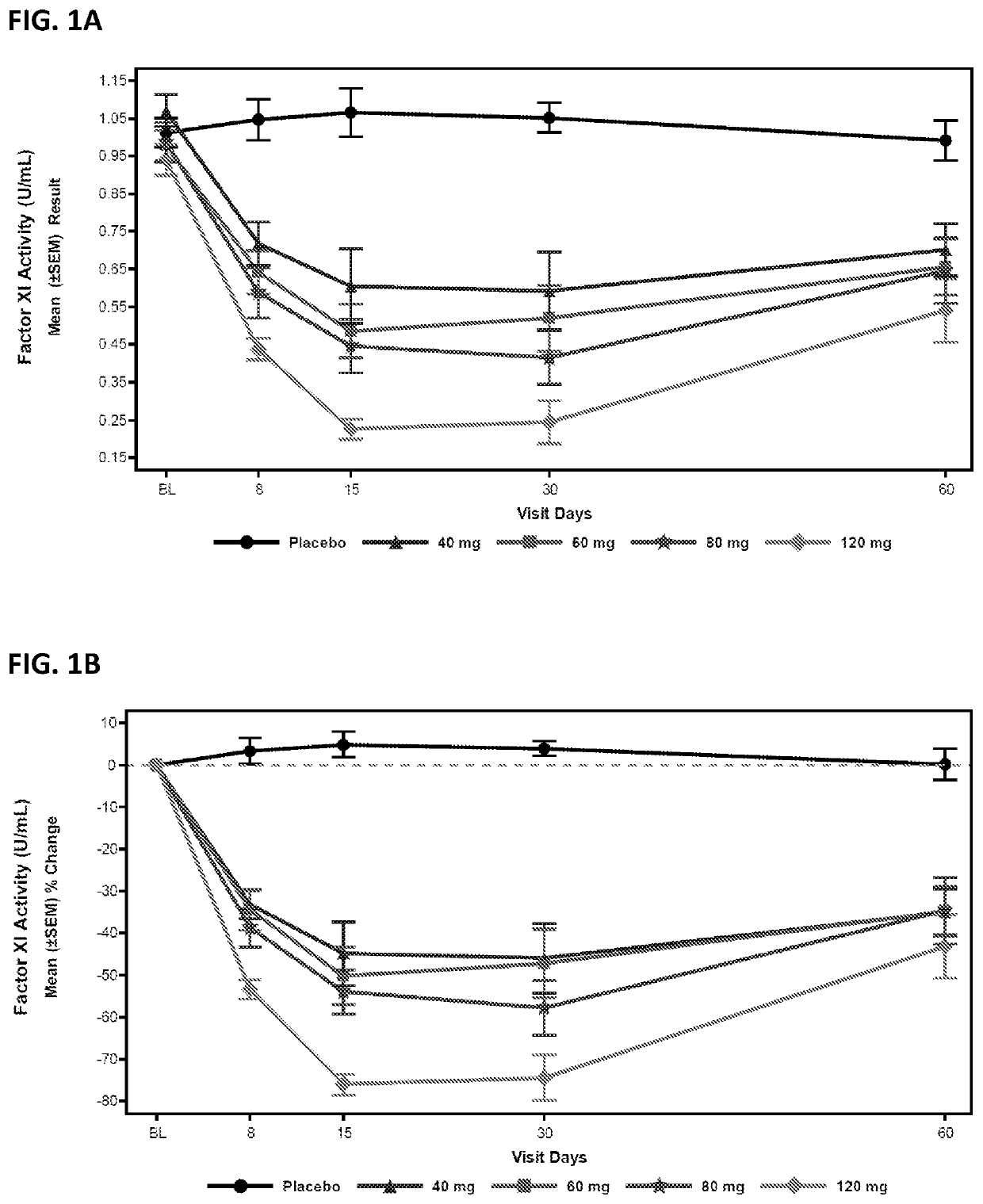
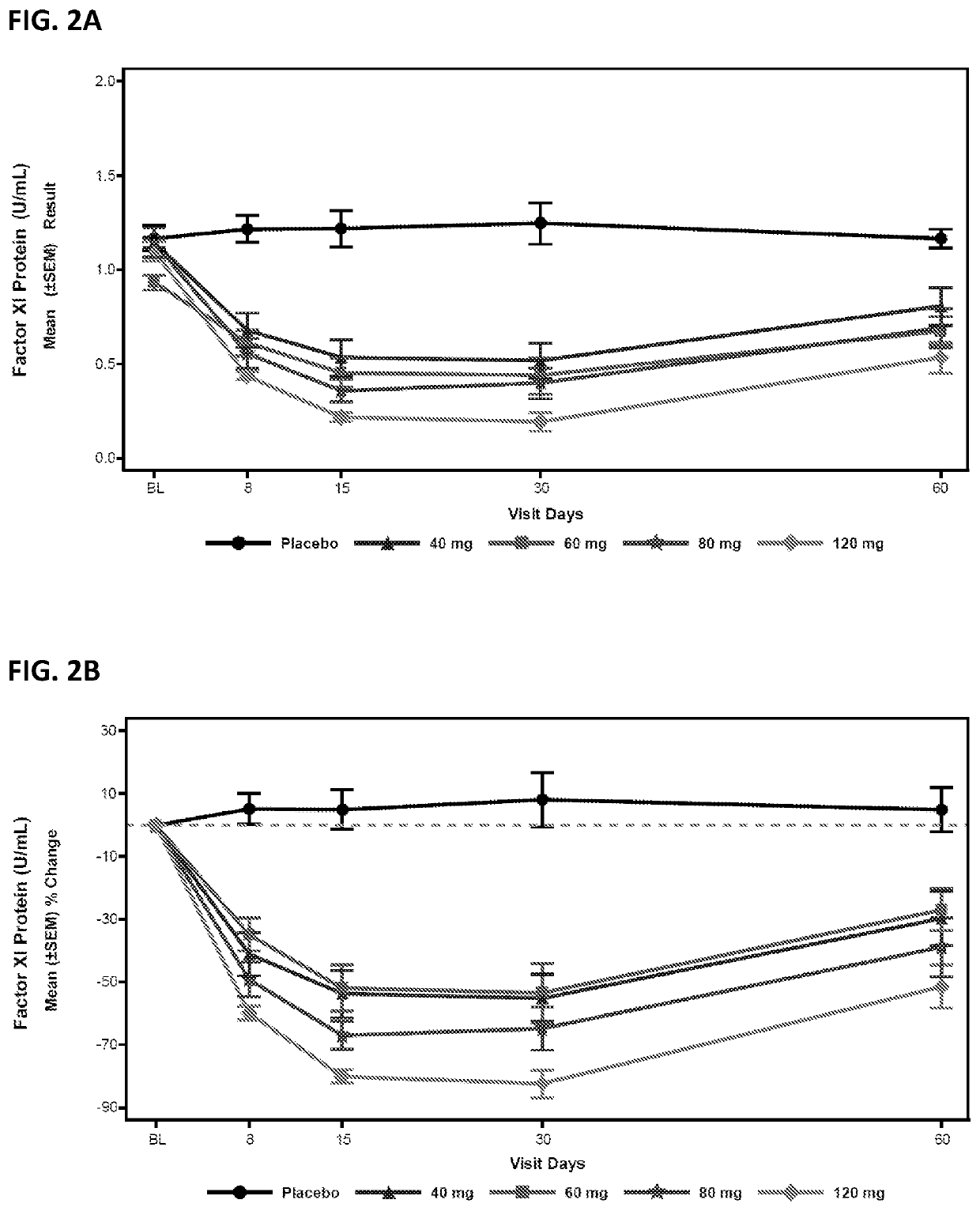
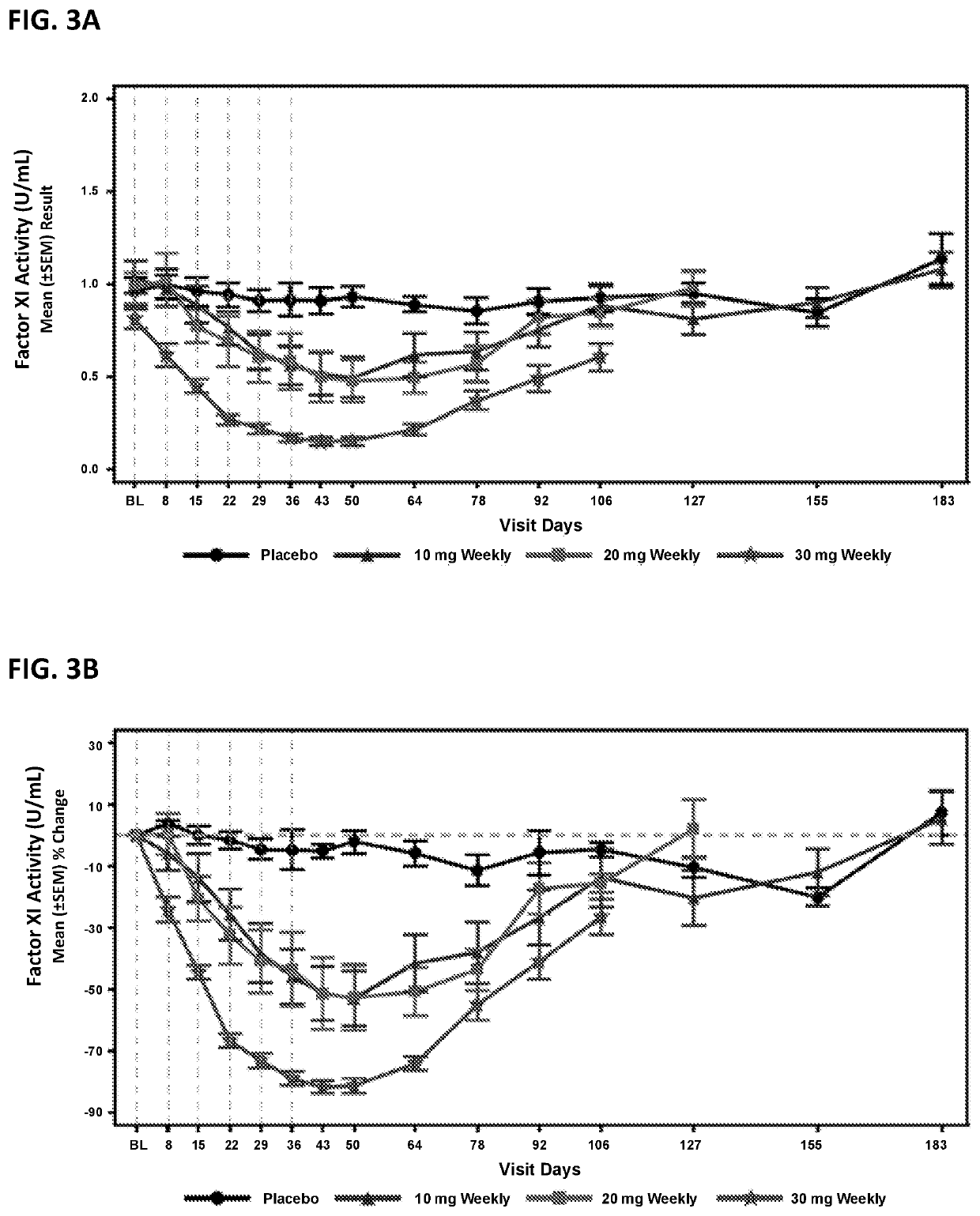
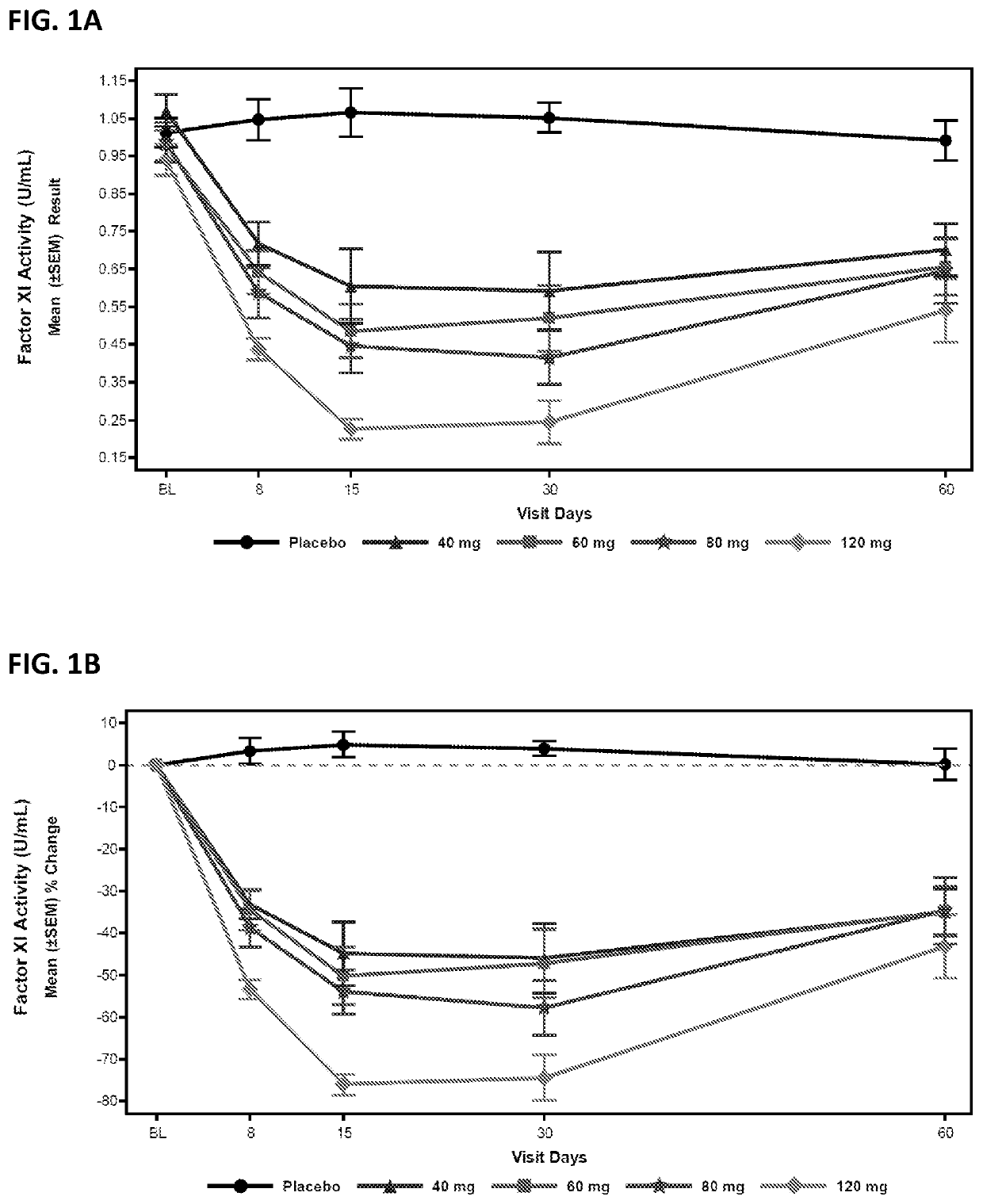
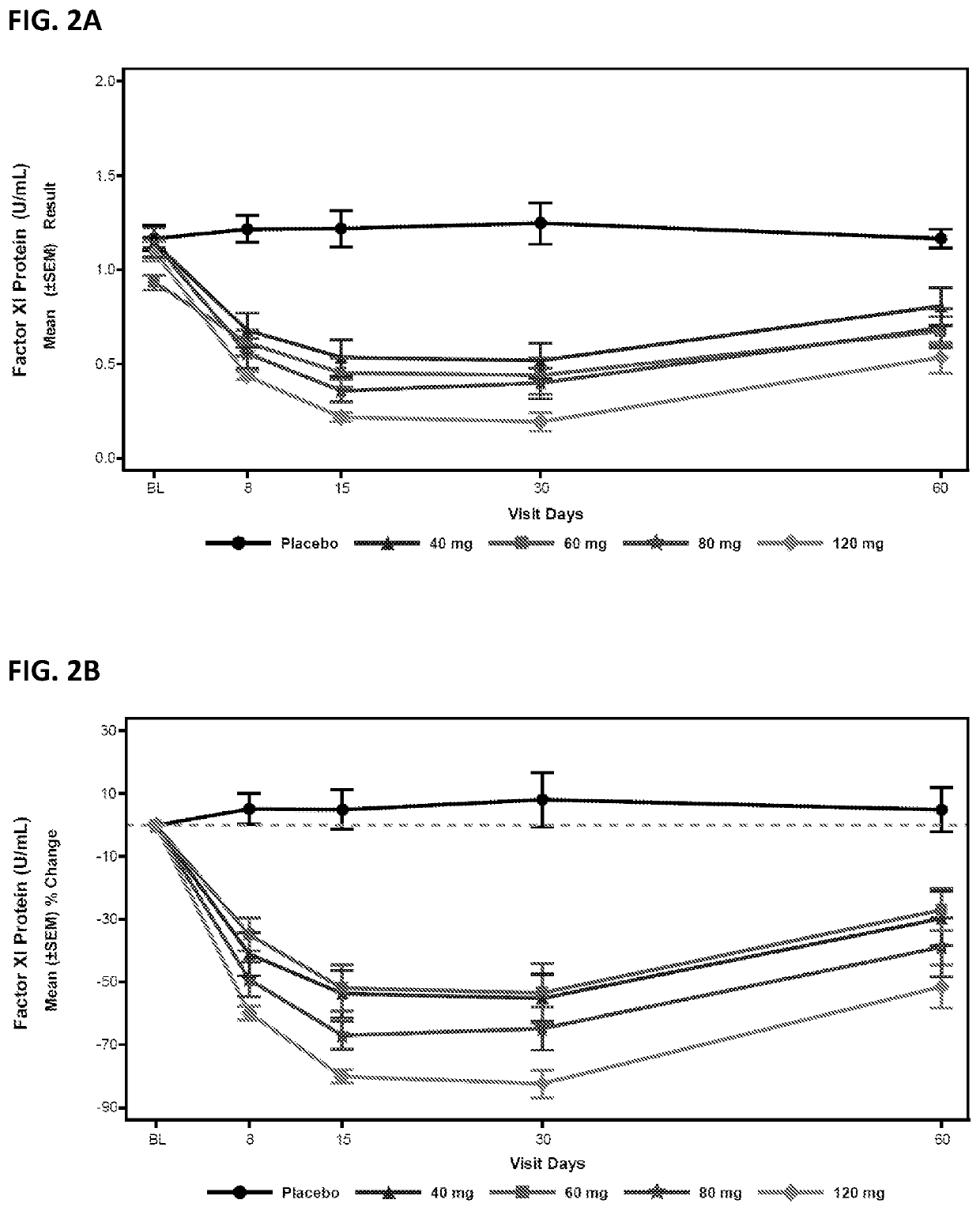
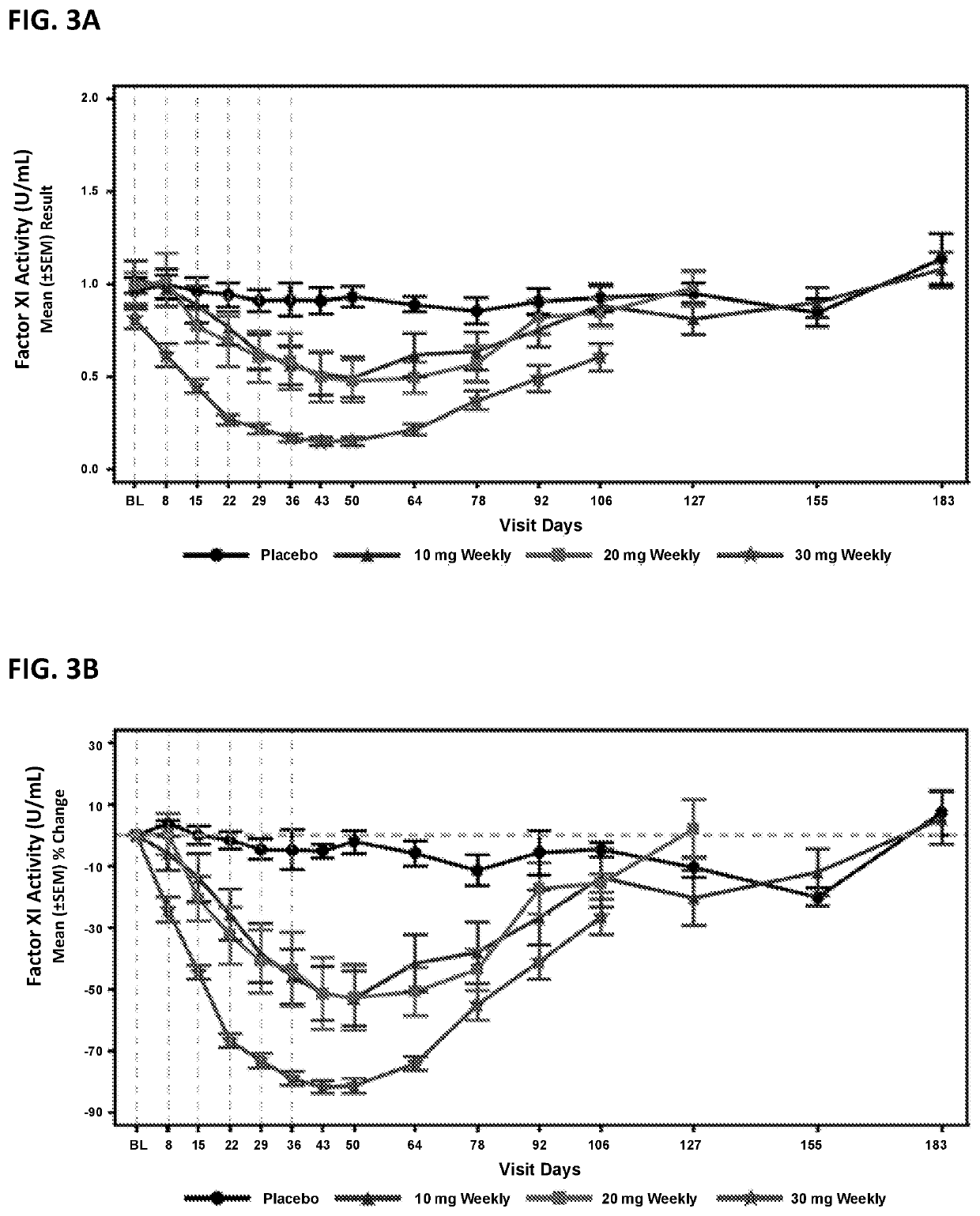
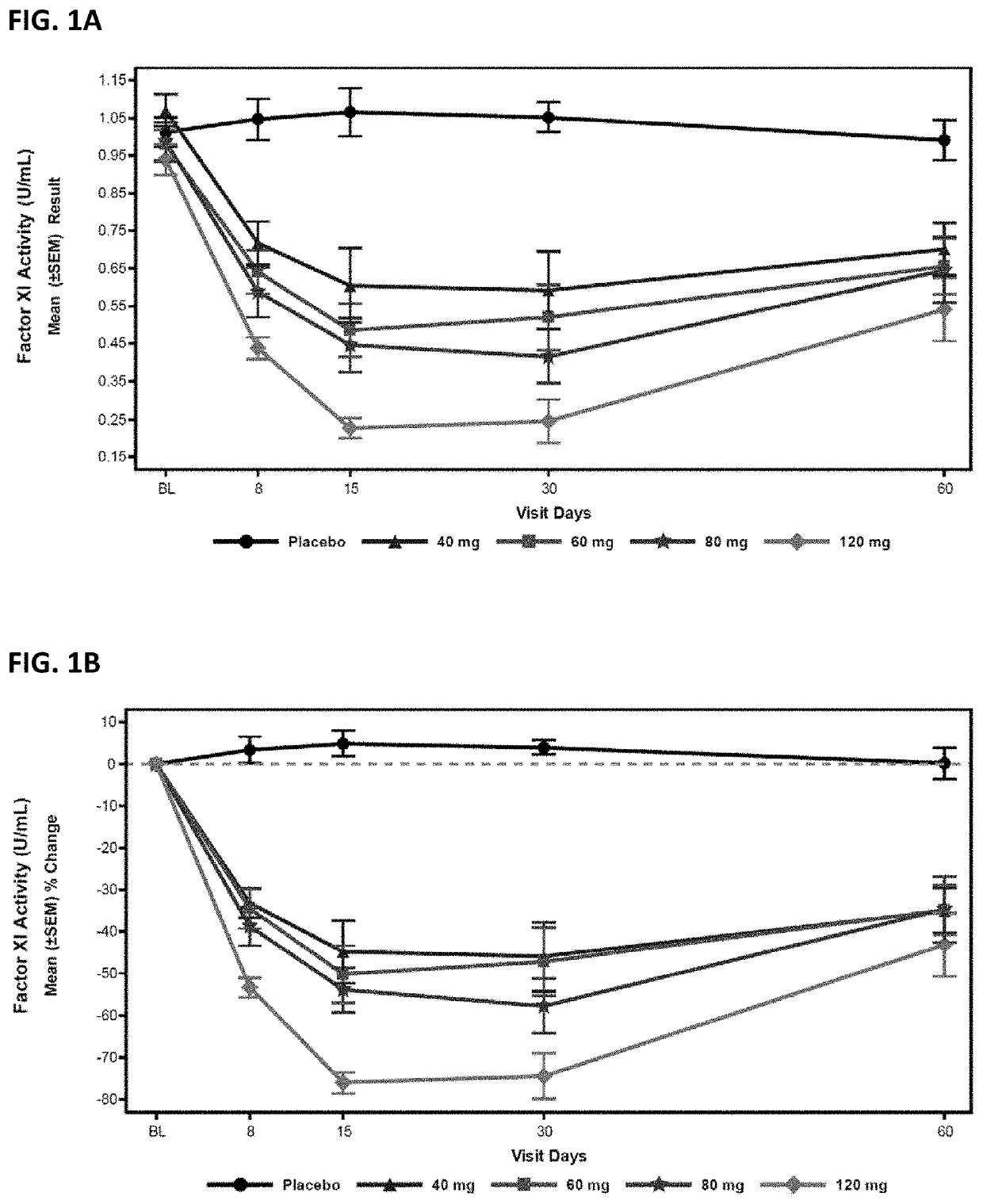
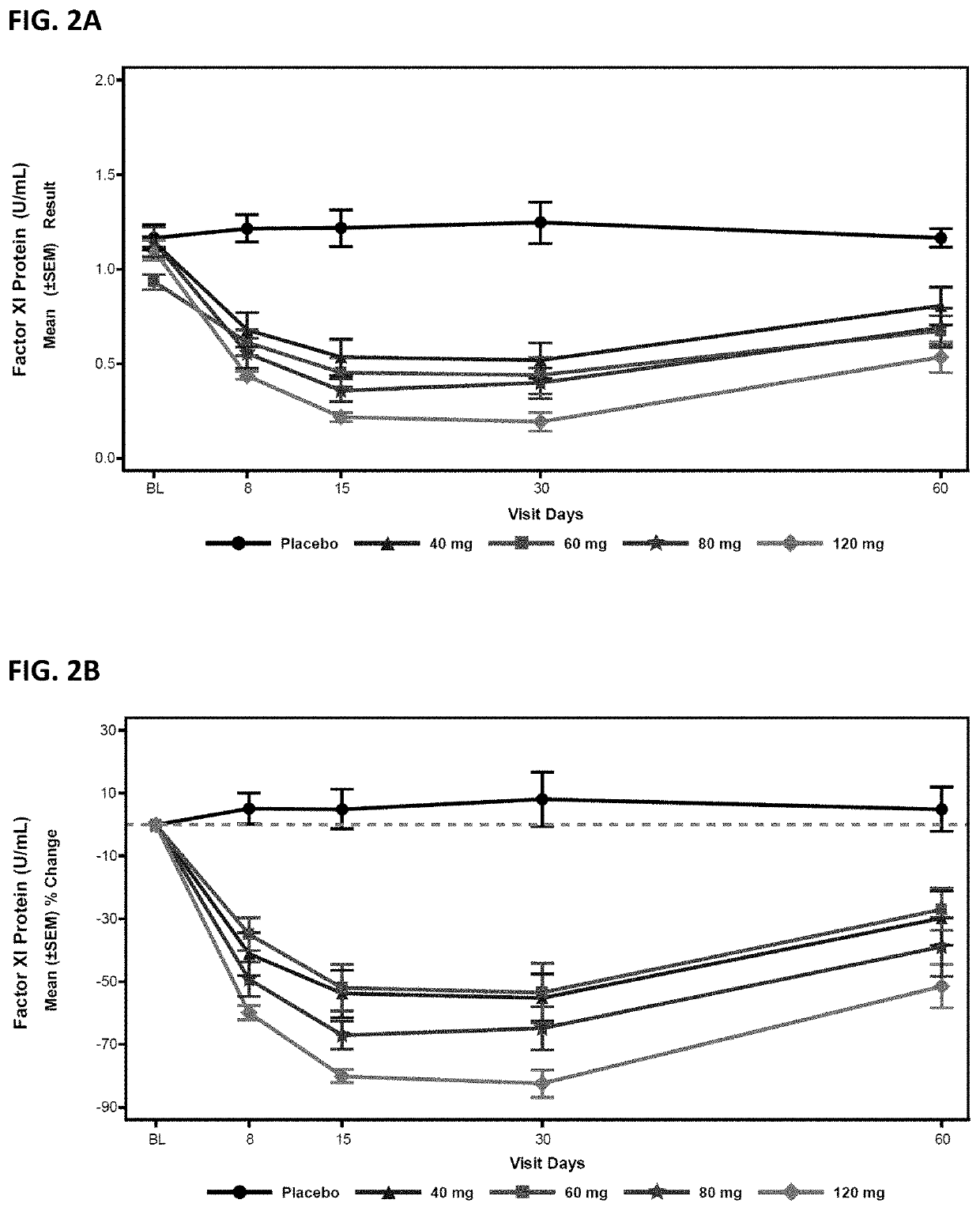
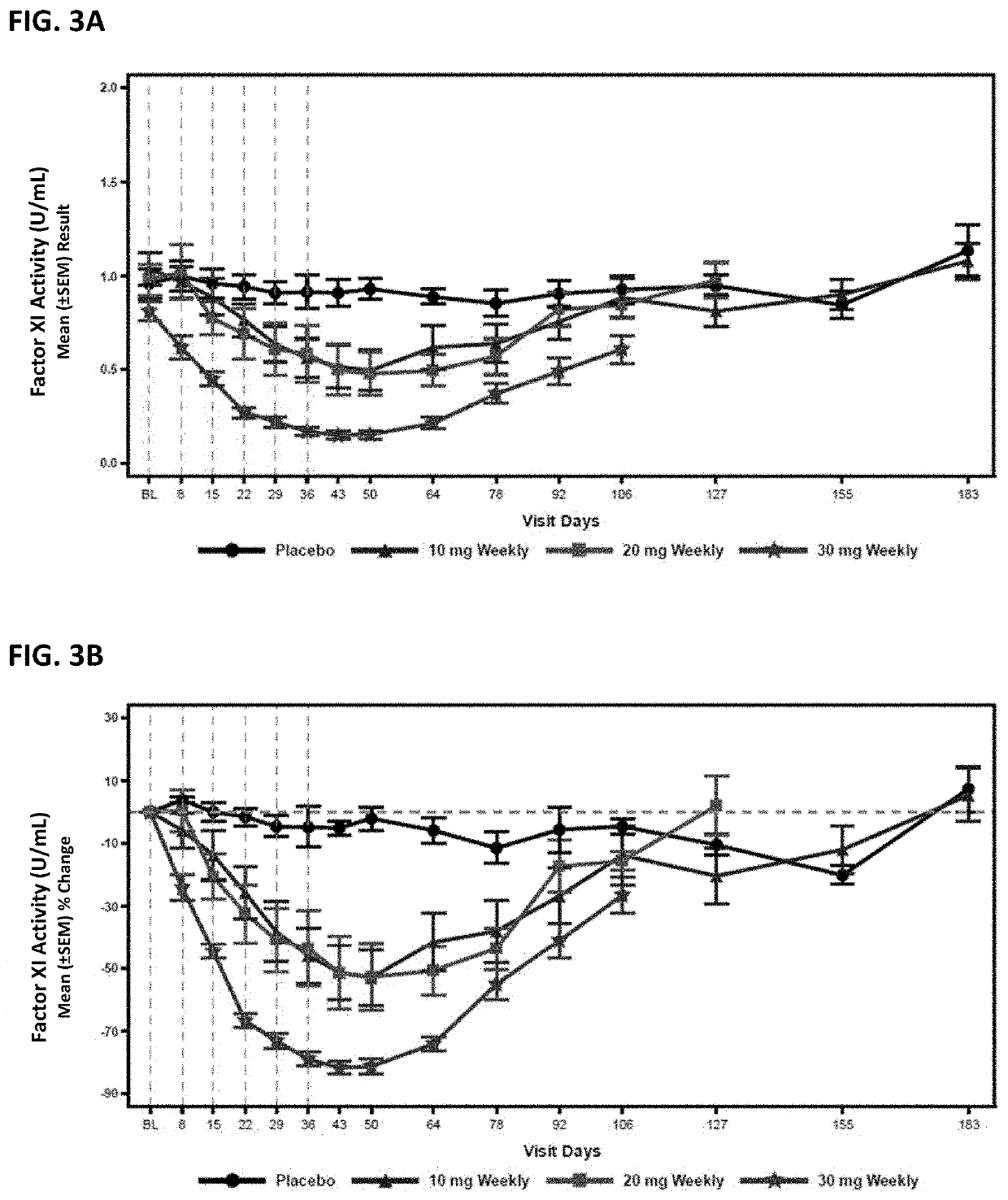
Compounds and methods for reducing fxi expression
ActiveUS20210087569A1Reduce the amount requiredReduce FXI protein activityCarbohydrate active ingredientsUrinary disorderDiseaseThrombus
Provided are compounds, methods, and pharmaceutical compositions for reducing the amount or activity of FXI RNA in a cell or subject, and in certain instances reducing the amount of FXI protein in a cell or subject. Such compounds, methods, and pharmaceutical compositions are useful to prevent, treat, or ameliorate at least one symptom of a thromboembolic condition without a significant increase in a bleeding risk. Such thromboembolic conditions include deep vein thrombosis, venous or arterial thrombosis, pulmonary embolism, myocardial infarction, stroke, thrombosis associated with chronic kidney disease or end-stage renal disease (ESRD), including thrombosis associated with dialysis, or other procoagulant condition. Such symptoms include decreased blood flow through an affected vessel, death of tissue, and death.
Owner:IONIS PHARMA INC
Dosing regimen of activated protein c and variants having reduced anticoagulant activity
InactiveUS20100284997A1Reduced anticoagulantReduce mortalityAntibacterial agentsHydrolasesDosing regimenLethal dose
Recombinant activated protein C (APC) and APC variants with reduced anticoagulant activity were used to reduce mortality in murine models of sepsis. These models included endotoxemia and bacteremia models. We discovered that single or multiple bolus doses of APC, especially of APC variants such as RR230 / 231AA-APC, KKK192-194AAA-APC and 5A-APC (containing the combination of mutations present in the first two APC variants) given as a single bolus reduces 7-day mortality of mice given lethal doses of endotoxin. Administrations of a single bolus of 5A-APC after the initiation of sepsis also reduces mortality caused by LPS. 5A-APC with ≦8% of normal anticoagulant activity (which has reduced risk of bleeding) reduces mortality when given as two bolus administrations at 3 hours and then at 10 hours after initiation of bacterial infection, i.e. after onset of sepsis. This shows, first, that one or more bolus injections of APC or of APC variants, especially 5A-APC, can reduce mortality when given beginning hours after the onset of sepsis and, second, that it is not necessary to administer APC as a continuous infusion which is the current standard of practice because one or more bolus administrations can reduce mortality. Furthermore, dosages of approximately 0.06 to 0.4 mg / kg of APC and APC variants are identified to be sufficient to reduce mortality in sepsis.
Owner:VERSITI BLOOD RES INST FOUND INC +1
Compounds and methods for reducing FXI expression
ActiveUS11021710B2Reduce riskDecreased blood flowCarbohydrate active ingredientsUrinary disorderDiseaseThrombus
Provided are compounds, methods, and pharmaceutical compositions for reducing the amount or activity of FXI RNA in a cell or subject, and in certain instances reducing the amount of FXI protein in a cell or subject. Such compounds, methods, and pharmaceutical compositions are useful to prevent, treat, or ameliorate at least one symptom of a thromboembolic condition without a significant increase in a bleeding risk. Such thromboembolic conditions include deep vein thrombosis, venous or arterial thrombosis, pulmonary embolism, myocardial infarction, stroke, thrombosis associated with chronic kidney disease or end-stage renal disease (ESRD), including thrombosis associated with dialysis, or other procoagulant condition. Such symptoms include decreased blood flow through an affected vessel, death of tissue, and death.
Owner:IONIS PHARMA INC
Hemostasis device for hepatobiliary surgery
InactiveCN112057218AAvoid bleedingConvenient careSurgeryMedical devicesSurgical operationDressing change
The invention relates to a hemostasis device, in particular to a hemostasis device for a hepatobiliary surgery. The situation that external force pulls an operative incision can be effectively prevented, automatical nursing and changing of medicines can be carried out, and the problem of reducing the physical burden of medical personnel when a compression hemostasis method is adopted during bleeding of the operative incision is solved. An arranged locking bleeding-preventing device can effectively prevent bleeding caused by pulling of the operative incision due to lateral movement of skin around a wound, an arranged dressing change device and a gauze fixing device can conveniently and rapidly nurse the operation incision, the operation incision can be healed benign and rapidly, and the bleeding risk is reduced. An arranged compression hemostasis device can be used for mechanically compressing the operation incision, medical workers do not need to pay too much physical strength, the medical workers have more energy to cope with other conditions, and the device can prevent bleeding and can further effectively cope with the bleeding condition.
Owner:HENAN PROVINCE HOSPITAL OF TCM THE SECOND AFFILIATED HOSPITAL OF HENAN UNIV OF TCM
Compounds and methods for reducing fxi expression
PendingUS20210355497A1Reduce riskDecreased blood flowCarbohydrate active ingredientsUrinary disorderDiseaseThrombus
Provided are compounds, methods, and pharmaceutical compositions for reducing the amount or activity of FXI RNA in a cell or subject, and in certain instances reducing the amount of FXI protein in a cell or subject. Such compounds, methods, and pharmaceutical compositions are useful to prevent, treat, or ameliorate at least one symptom of a thromboembolic condition without a significant increase in a bleeding risk. Such thromboembolic conditions include deep vein thrombosis, venous or arterial thrombosis, pulmonary embolism, myocardial infarction, stroke, thrombosis associated with chronic kidney disease or end-stage renal disease (ESRD), including thrombosis associated with dialysis, or other procoagulant condition. Such symptoms include decreased blood flow through an affected vessel, death of tissue, and death.
Owner:IONIS PHARMA INC
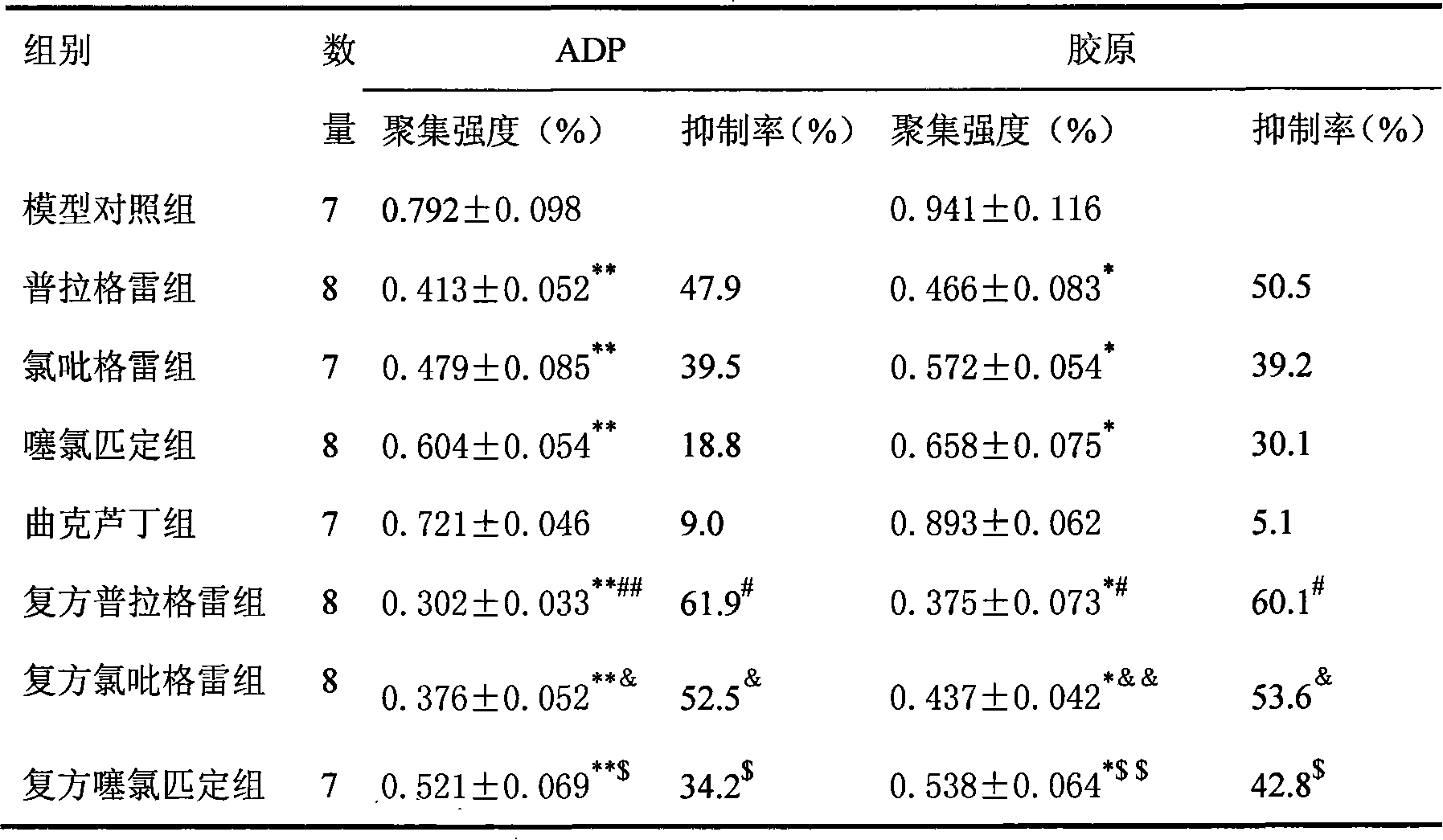
Drug composite containing troxerutin and ADP receptor antagonist
ActiveCN101637477AEasy to acceptEasy to carryOrganic active ingredientsBlood disorderAnticoagulation ActivityNK1 receptor antagonist
The invention belongs to a new drug composite, in particular to a drug composite which adopts troxerutin and ADP receptor antagonist as active ingredients. In order to find a composite drug compositefor curing thrombotic diseases which can not only keep the fast and forceful anticoagulation activity, but also effectively reduce the bleeding risk, the inventor finds that using both troxerutin andADP receptor antagonist for inhibiting the thrombosis has unexpected effect and extremely good synergistic effect through serious and repeated experiments and the mixed use of the both drugs can not only increase the antithrombotic function, but also reduce the bleeding risk when using ADP receptor antagonist so that the use of both drugs has better and more effective anticoagulation activity andfaster function.
Owner:LUNAN PHARMA GROUP CORPORATION
Upper limb stabilizer and radial artery compression assembly
PendingCN114767204AReduce bleeding riskUpper extremity hematoma prevention and reductionSurgeryTherapeutic coolingBrachial arteryVein
The invention provides an upper limb stabilizer and a radial artery compression assembly, relates to the technical field of medical instruments, and solves the technical problem that in the prior art, a radial artery compressor cannot prevent and treat upper limb swelling caused by radial artery approach puncture. The upper limb stabilizer at least comprises a first pressing piece, a second pressing piece and a cooling piece which are arranged in parallel, the cooling piece is arranged between the first pressing piece and the second pressing piece, and the first pressing piece and the second pressing piece can be pressed at the brachial artery position of an upper limb. The upper limb bleeding risk can be greatly reduced and the stability of the system can be improved by performing targeted compression on the brachial artery, and when the compression point of the radial artery fails, the first compression piece and the second compression piece at the brachial artery can effectively and temporarily reinforce compression on the upper limb vein path, so that the occurrence of upper limb hematoma can be greatly prevented; the cooling piece is additionally arranged between the second pressing piece and the second pressing piece, so that the hematoma range of the upper limbs can be remarkably prevented and reduced.
Owner:苗润静
Novel bellyband used after kidney puncture operation
PendingCN113967129AReduce bleeding riskAvoid bleedingBreast bandagesDevices for pressing relfex pointsEngineeringMechanical engineering
The invention relates to a novel bellyband used after a kidney puncture operation. The novel bellyband comprises a left wing part, a middle section part, a right wing part and an overlapping part which are connected in sequence; the outer end part of the left wing part is provided with a bonding part corresponding to the overlapping part; the middle parts of the left wing part and the right wing part are respectively provided with an inflating bag which is used for pressing and is provided with an inflating catheter; and a traditional Chinese medicine patch is arranged in the middle of the middle section part, and a small air bag with an inflation catheter is arranged below the traditional Chinese medicine patch and used for stimulating acupuncture points. A doctor only needs to inflate and deflate to achieve the purposes of compression, recompression and compression relief according to the illness condition, and the risk of hemorrhage after renal puncture caused by changing the body position of a patient or improperly exerting force is reduced; and the traditional Chinese medicine is combined, acupoint stimulation and traditional Chinese medicine external application are utilized, urination is promoted, anxiety and discomfort caused by dysuria are reduced, and catheterization is avoided.
Owner:ZHONGSHAN HOSPITAL FUDAN UNIV
Superfine thoracoscope
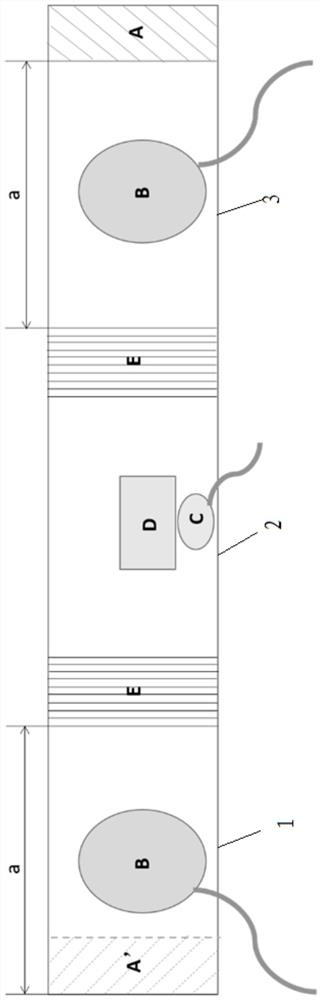
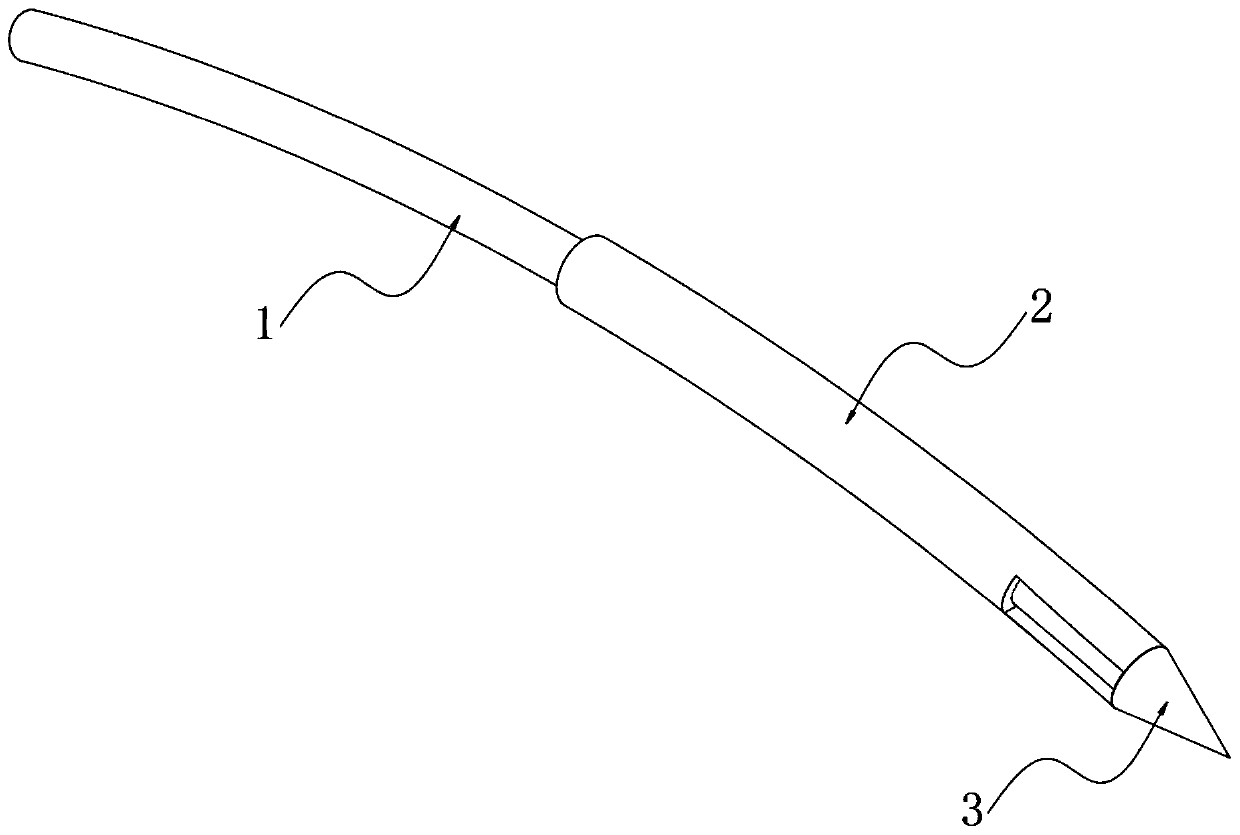
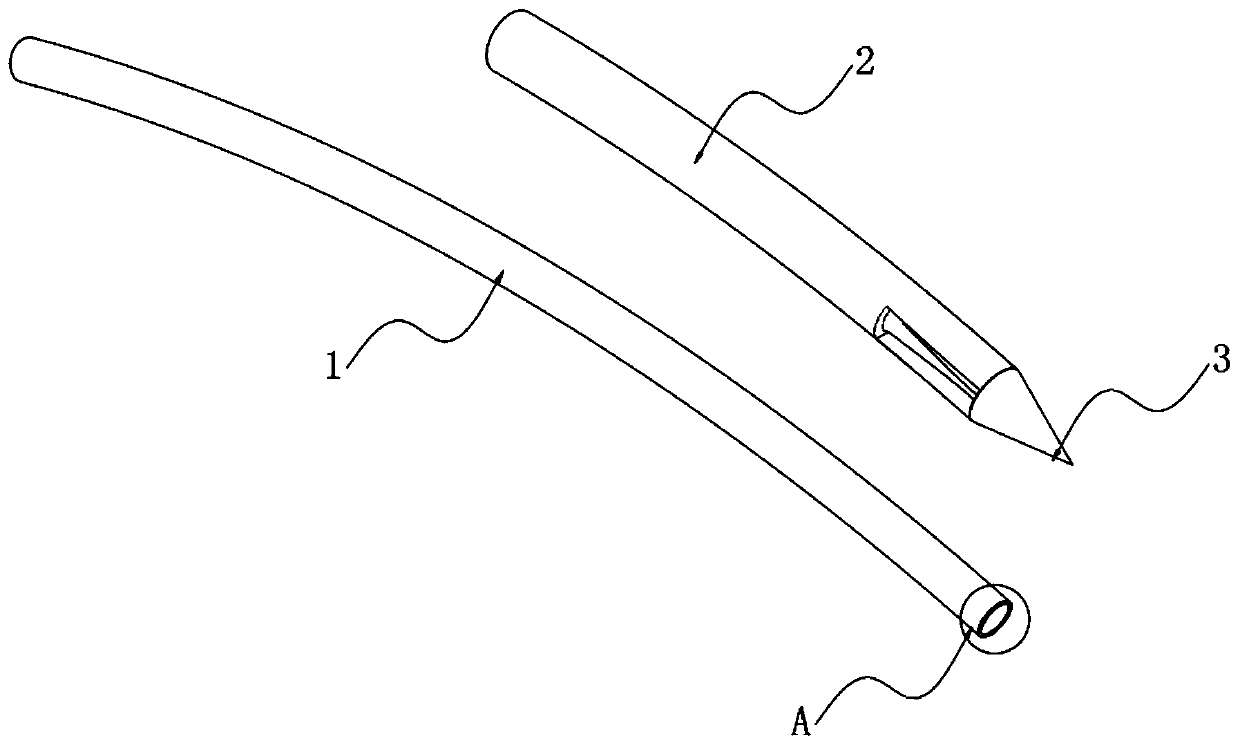
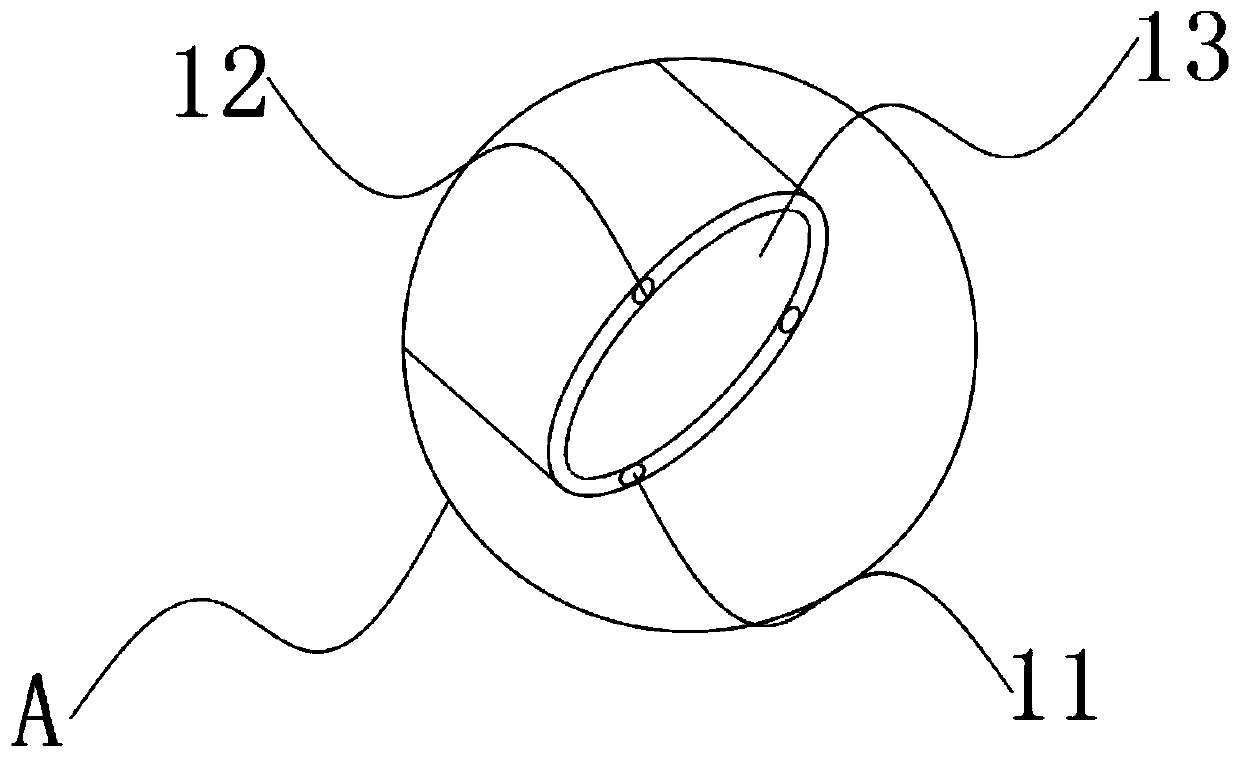
InactiveCN110833380ANot easy to damageAvoid damageSurgical needlesEndoscopesEngineeringThoracoscopes
The invention relates to the technical field of thoracoscopes, in particular to a superfine thoracoscope, which comprises a thoracoscope body, the thoracoscope body is sleeved with a sleeve, and a blunt puncture needle is embedded in the end of the thoracoscope body. A biopsy suction hole channel is formed in the endoscope body, two symmetrical glass fiber light source beams are further embedded in the endoscope body, and an image collecting hole is further formed in one side of the biopsy suction hole channel. According to the superfine thoracoscope, the blunt puncture needle with the sleevepenetrates into the thoracic cavity, intercostal blood vessels and nerves are not prone to being damaged, and the risk of bleeding is avoided; meanwhile, the mirror bodies with two radians are arranged, so that the thoracic wall focus and biopsy are convenient to observe, and far and near focuses are convenient to detect; in addition, compared with a channel formed by traditional puncture, a puncture channel formed by the arrangement is small in damage, suture is not needed after operation, the compliance of a patient is improved, and the problem that an existing thoracoscope for the internalmedicine department is prone to causing obvious trauma and bleeding risks is solved.
Owner:上海市闵行区中心医院
Identification of thrombosis or bleeding risk in an individual with and without Anti-platelet therapy
InactiveUS20140179766A1Prevent uncontrolled cell proliferationImprove stabilityBioreactor/fermenter combinationsBiocideMajor bleedingBleeding complication
Owner:ROYAL COLLEGE OF SURGEONS & IRELAND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com