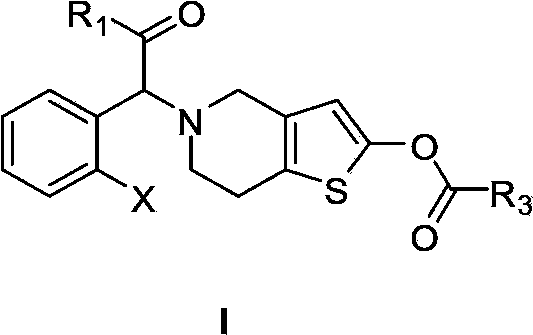
Thienopyridine derivatives, and preparation methods and medical use thereof
A use and drug technology, applied in the field of thiophenepyridine derivatives and their preparation, can solve the problems of cardiovascular events and increased mortality, and achieve the effects of good anti-platelet aggregation effect, solving resistance problems and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
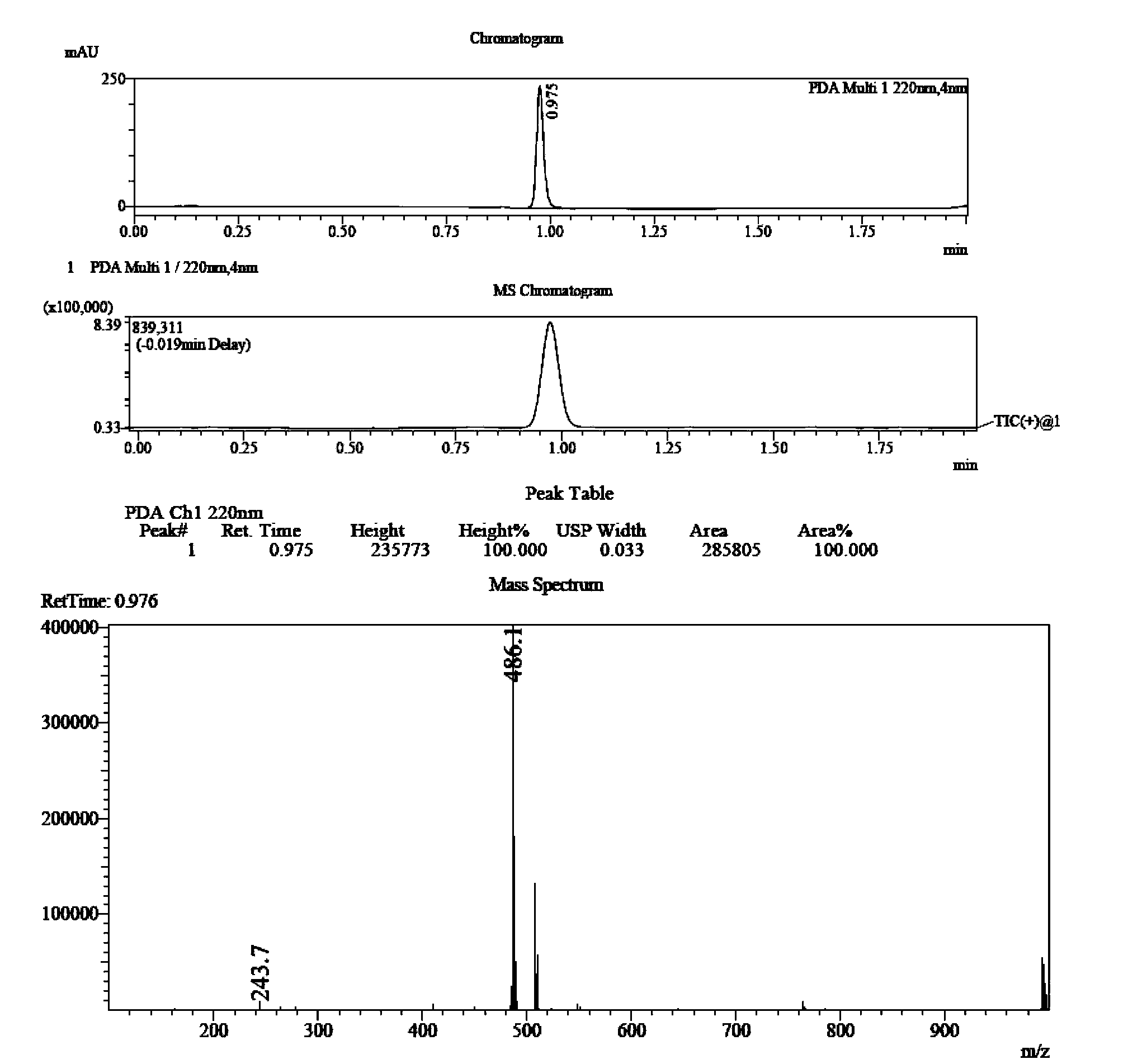
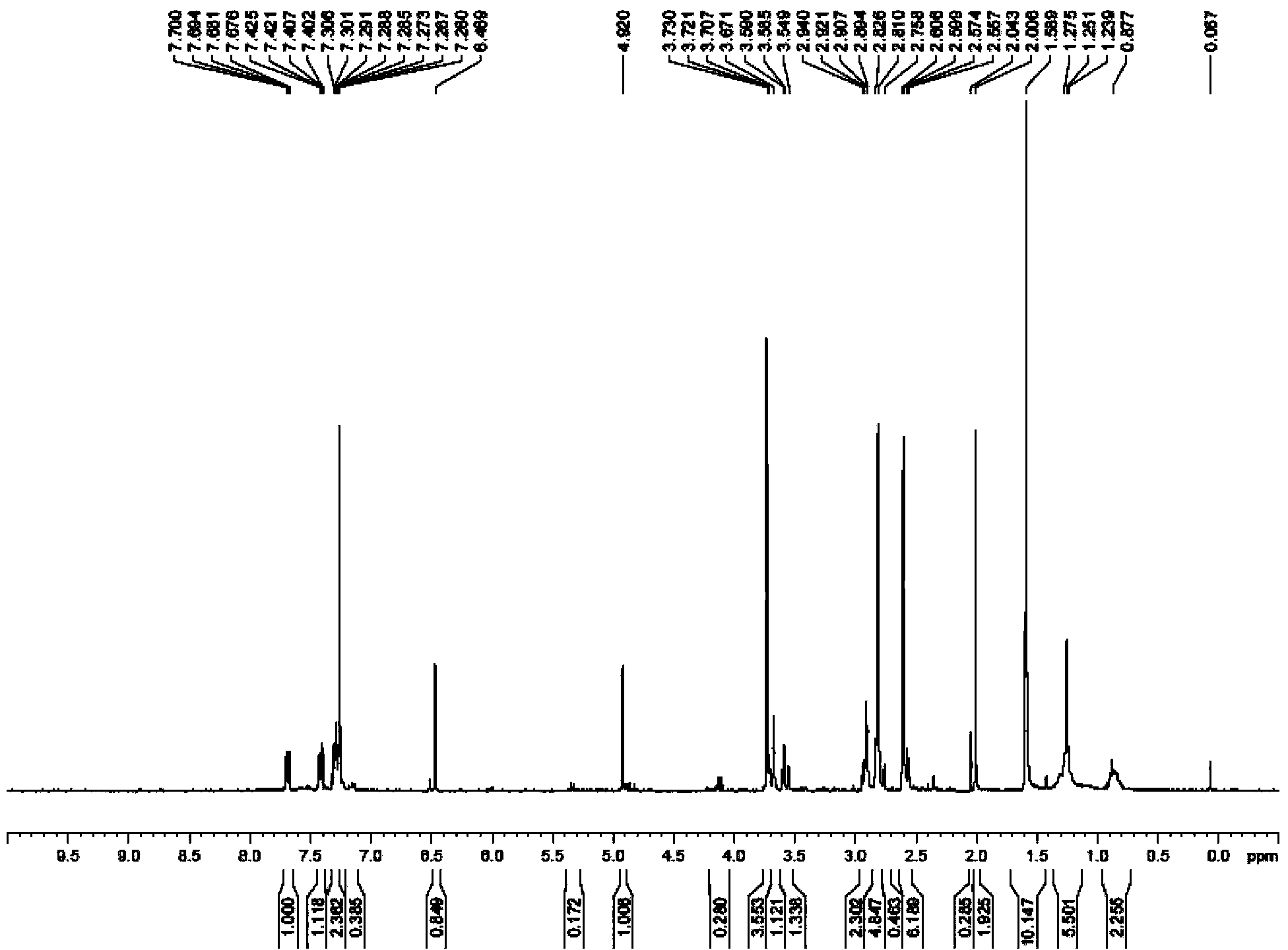
Embodiment 1
[0068] 2-(3,5,6-Trimethylpiperazineformyloxy)-2-(2-chlorophenyl)-2-(6,7-dihydrothieno[3,2-C]pyridine- 5-base) the preparation method of methyl acetate, its synthetic route is as follows:
[0069]
[0070] Dissolve QR02000-IN-07 (10mmol, 1.0eq) in 20mL methanol, add QR02000-IN-08 (10mmol, 1.0eq) and NaHCO 3 (20mmol, 2.0eq), heated to 70°C, reacted for 6h. Cool to room temperature, filter to remove inorganic salts, evaporate the solvent under reduced pressure, add 100mL ethyl acetate and 30mL water, separate the organic layer, wash the organic layer twice with water, dry over anhydrous sodium sulfate, remove the solvent under reduced pressure, and use a flash column The product QR02000-IN-09 was purified.
[0071] Add thionyl chloride (60mmol, 2.0eq) dropwise to 60mL of dichloromethane solution in which QR02000-IN-02 (30mmol, 1.0eq) was dissolved in ice-cooling, add to room temperature and stir for 2h, evaporate to dryness under reduced pressure The product acid chloride w...
Embodiment 2
[0073] 2-(3,5,6-Trimethylpiperazineacetoxy-2-(2-chlorophenyl)-2-(6,7-dihydrothieno[3,2-C]pyridine-5- Base) the preparation of methyl acetate:
[0074]
[0075] Add oxalyl chloride (60mmol, 2.0eq) dropwise to 60mL of dichloromethane solution in which QR02000-IN-03 (30mmol, 1.0eq) was dissolved in an ice bath. in anhydrous dichloromethane; add this solution dropwise to a solution of QR02000-IN-09 (15mmol, 0.5eq) and triethylamine (180mmol, 3.0eq) in dichloromethane at 0°C, add After the completion, the temperature of the reaction system was controlled at about 0° C., the stirring was continued for 0.5 hours, and the temperature was raised to room temperature and stirred for 2 hours. Pour the reaction solution into 60 mL of ice water, extract with ethyl acetate (100 mL×3), combine the organic phases, wash the organic phases with saturated brine, dry over anhydrous sodium sulfate, concentrate and evaporate to dryness, and obtain the target product QR02002 by flash column chrom...
Embodiment 3
[0077] 2-(3,4,5-trihydroxycyclohexenecarboxy)-3,5,6-trimethylpiperazineacetoxy-2-(2-chlorophenyl)-2-(6, Preparation of 7-dihydrothieno[3,2-C]pyridin-5-yl)methyl acetate:
[0078]
[0079] Oxalyl chloride (60mmol, 2.0eq) was added dropwise to a solution of QR02000-IN-05 (30mmol, 1.0eq) and triethylamine (120mmol, 3.0eq) in 60mL of dichloromethane solution in an ice bath, and the addition was completed to Stir at room temperature for 2h, concentrate to dryness, and then dissolve in anhydrous dichloromethane; add the solution dropwise to a solution of QR02000-IN-09 (15mmol, 0.5eq) and triethylamine (180mmol, 3.0 In the dichloromethane solution of eq), after the addition, the temperature of the reaction system was controlled at about 0° C., and the stirring was continued for 0.5 hours, and the temperature was raised to room temperature and stirred for 2 hours. The reaction solution was poured into 60 mL of ice water, extracted with ethyl acetate (100 mL×3), the organic phases ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com