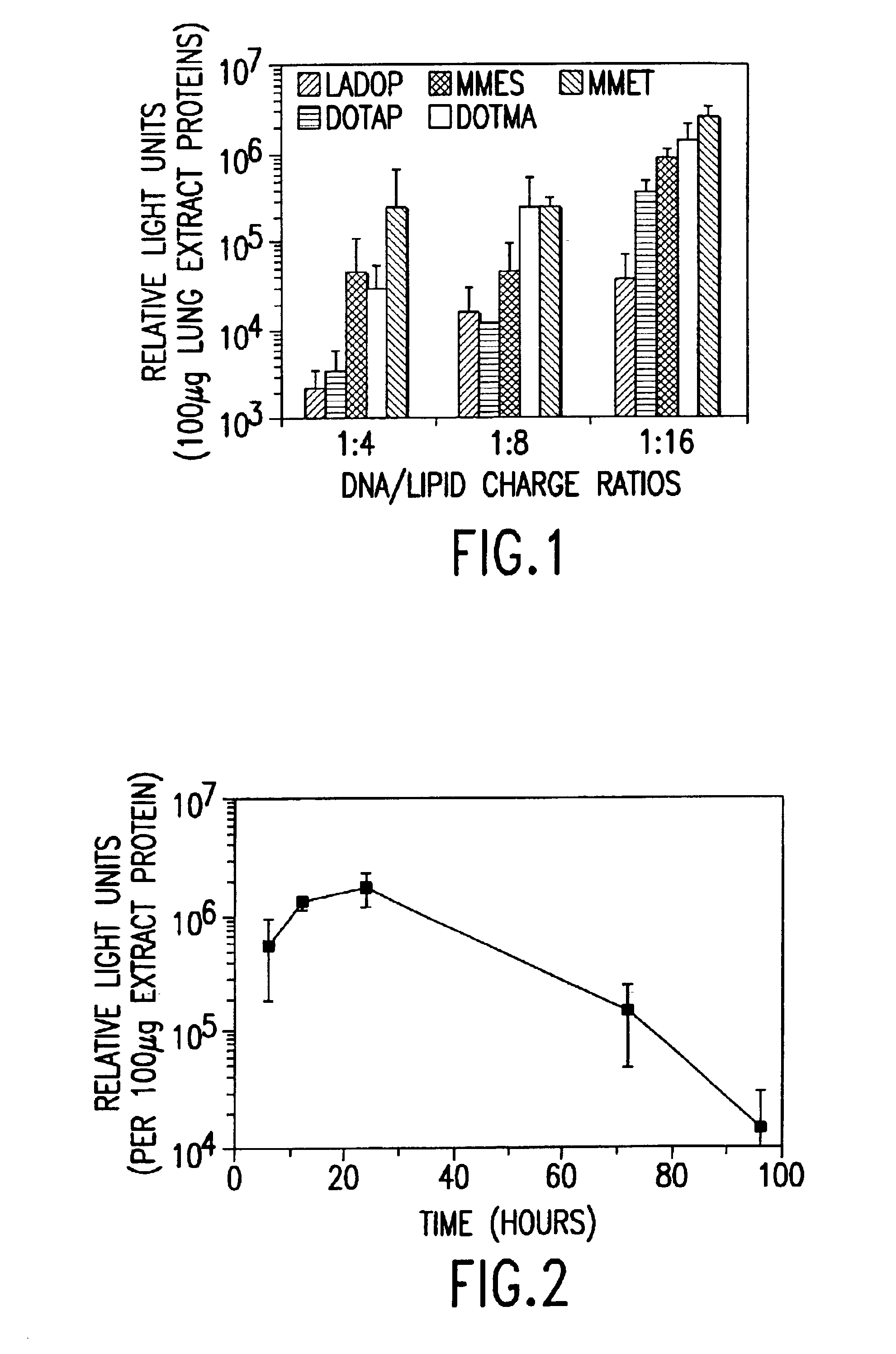
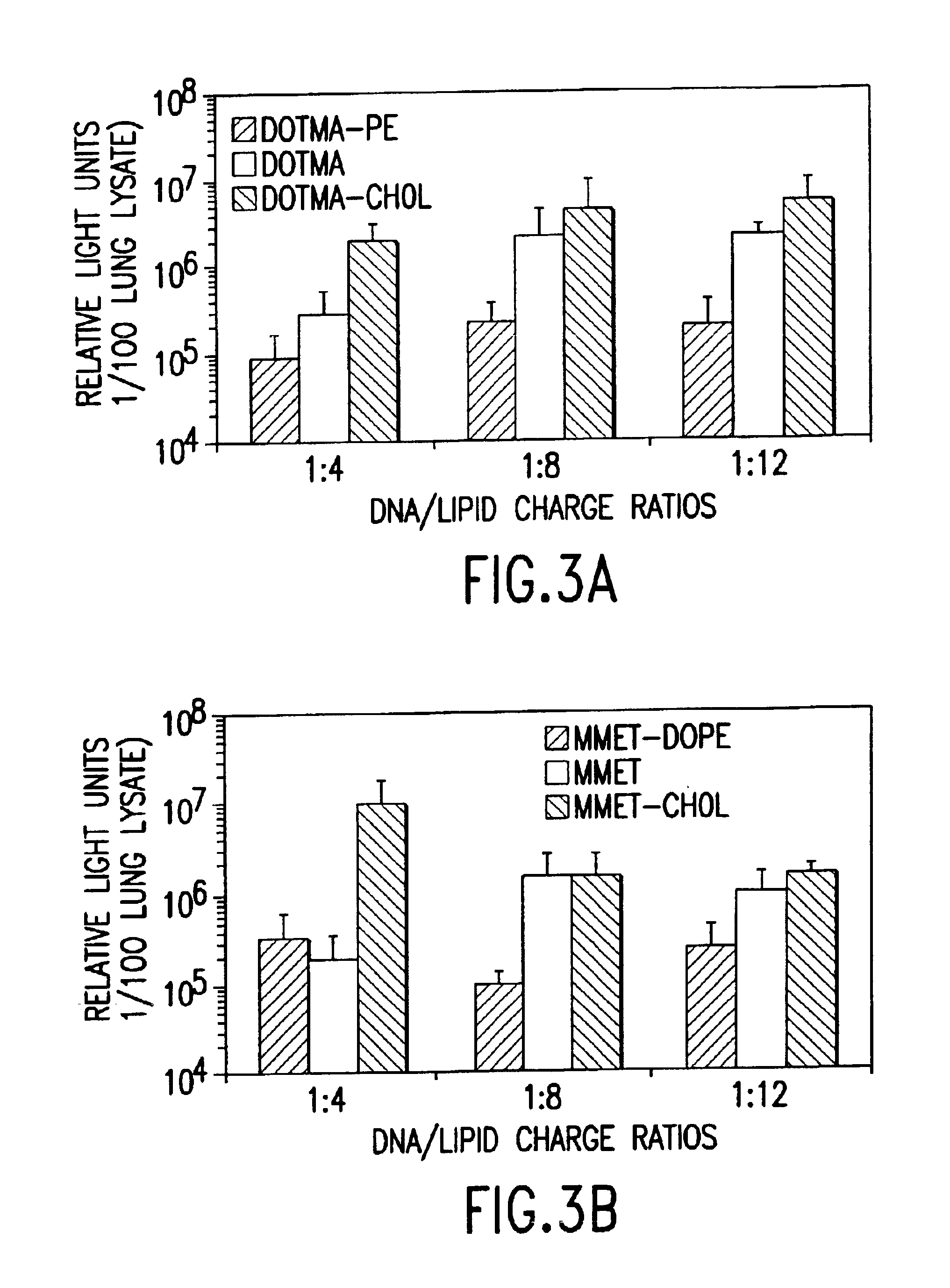
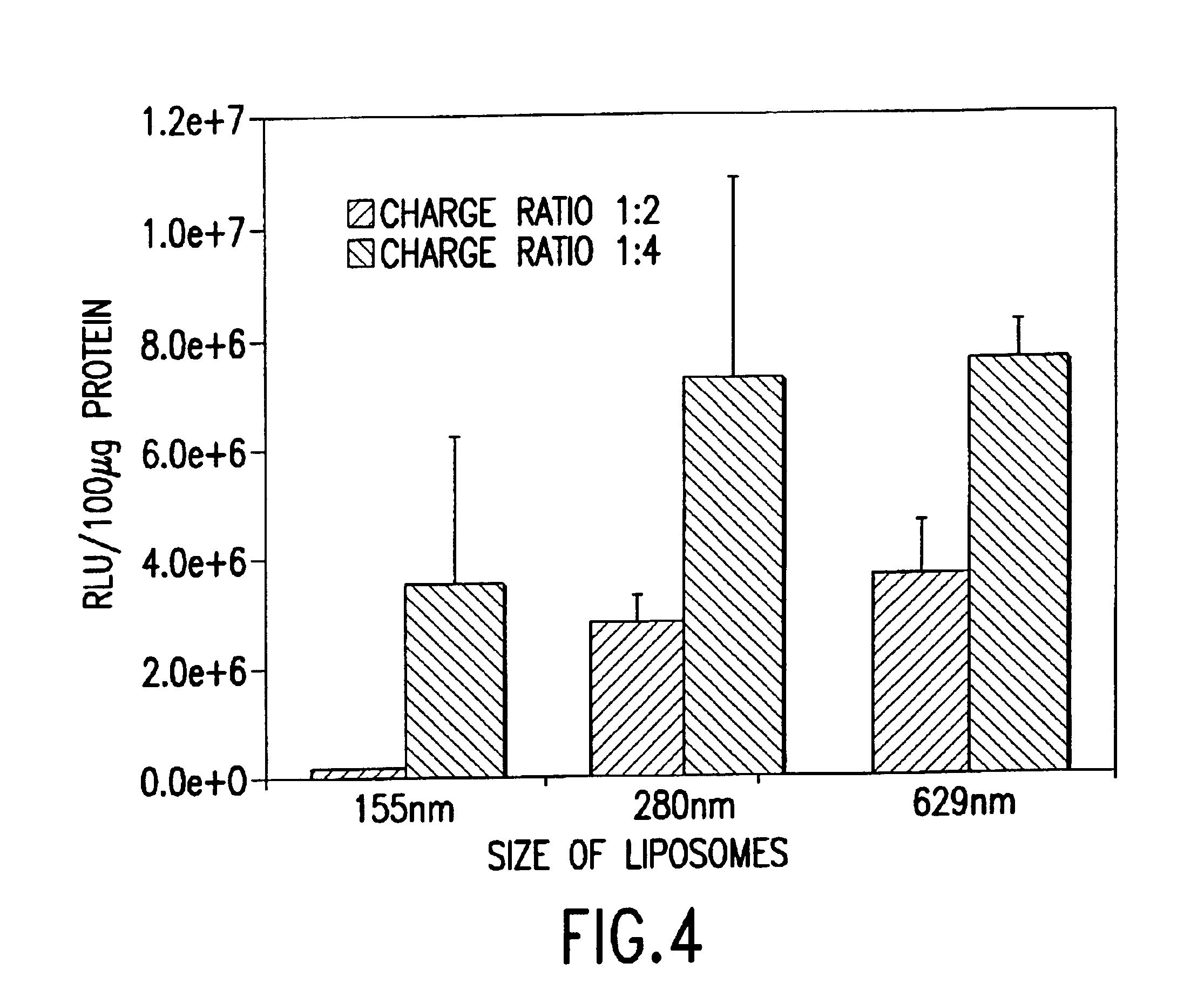
Cationic liposomes for gene transfer
a technology of cationic liposomes and gene transfer, which is applied in the direction of organic chemistry, chemical apparatus and processes, and material ingredients, etc., can solve the problems of varying degrees of lipid/dna treatment animals, strong inhibition of transfection efficiency, and increased toxicity of lipid/dna treated animals, so as to ensure stability in solution, good membrane fluidity, and minimal toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0189]Synthesis of LADOP:
[0190]To a solution of 0.68 g (7.5 mmole) (+ / −)-3-amino-1,2,-propanediol in 20 ml dry methanol, 1.1 ml (8 mmole) triethylamine (TEA) in 10 ml methanol was added. At room temperature, a solution of 3.1 g (7 mmole) N,N-diBoc-lysine-N-hydroxysuccinimide ester in CH2Cl2 (20 ml) was added. The reaction was allowed to continue for 1 hour, after which the solvents were evaporated. The solid was dissolved in 100 ml CHCl3 and washed with brine (200 ml) twice. The organic phase was collected and dried over sodium sulfate and the solvent was evaporated. The product was purified using 40 g silica gel to give diBoc-lysyl3-amino-1,2-propandiol 2.51 g (5.54 mmole). To a solution of 0.65 g (1.5 mmole) diBoc-lysyl3-amino-1,2-propandiol in dry CH2Cl2, 1 ml TEA and 1 ml oleic chloride (3 mmole) was added. The reaction was allowed to continue for 4 hrs. After routine work up, the product was purified with 20 g silica gel. The Boc protecting groups were then removed by trifluoro...
example 2
[0192]Synthesis of MMET:
[0193]To a solution of 3-N-morpholino-1,2-propandiol (161 mg, 1 mmole) in 5 ml DMSO 0.4 g KOH was added. After 20 minutes, 1 g (3 mmoles) of oleyl methanesulfonate in 2 ml DMSO was added. The reaction was performed under Ar2 at room temperature for 20 h. The reaction mixture was washed with brine in hexane and purified with silica gel. The yeild of 1,2-dioleyl-3-N-morpholino-1,2-propane was 540 mg as colorless oil. To convert 1,2-dioleyl-3-N-morpholino-1,2-propane to MMET, 330 mg of 1,2-dioleyl-3-N-morpholino-1,2-propane (0.5 mmole) was dissolved in 5 ml methanol to which 500 ul of CH3I was added. The reaction was performed at room temperature at dark. The organic solvents were removed by a rotovapor. The residue were dissolved in 100 ml CHCl3 and washed with brine. The crude product was purified over silica gel to give 300 mg final product. NMR data: chemical shift: 0.84 (t, CH3, 6H); 1.23 (s, —CH2-, 44H); 1.51 (m, CH2C—O, 4H); 2.03 (m CH2C═, 8H), 3.40 (m, C...
example 3
[0194]Synthesis of N-(3-dioctadecylaminopropyl)-N′,N′-bis(lysyl-epsilon-lysyl)-L-lysinamide:
[0195]Synthesis of N,N-dioctadecyl-3-propyldiamine: To a suspension containing 2 g of dioctadecylamine (4 mmole), in 20 ml methanol and 20 ml CH2CL2, was added 20 ml CH2=CHCN. The reaction was contineud for 24 h at room temperature. The solvents were removed under vacuum and the product was purified with silica gel. The product was then dissolved in 20 ml ether and 2 g of LiAlH4 was added. The reduction was allowed for overnight at room temperature. The reaction was stoped with dilute NaOH in water at 0 C. The reaction mixture was filtered. The organic solvent was dried over sodium sulfate and evaporated to give 2.3 g white powder. NMR data (CDCl3): chemical shift 0.86 (t, CH3 8H); 1.24 (64H, Ch2, s), 1.49 (2H, N-C—CH2—C—N—, m); 1.59 (2H, NH2, m) 2.39 (6H, CH2N, m); 2.70 (2H, CH2N, t).
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com