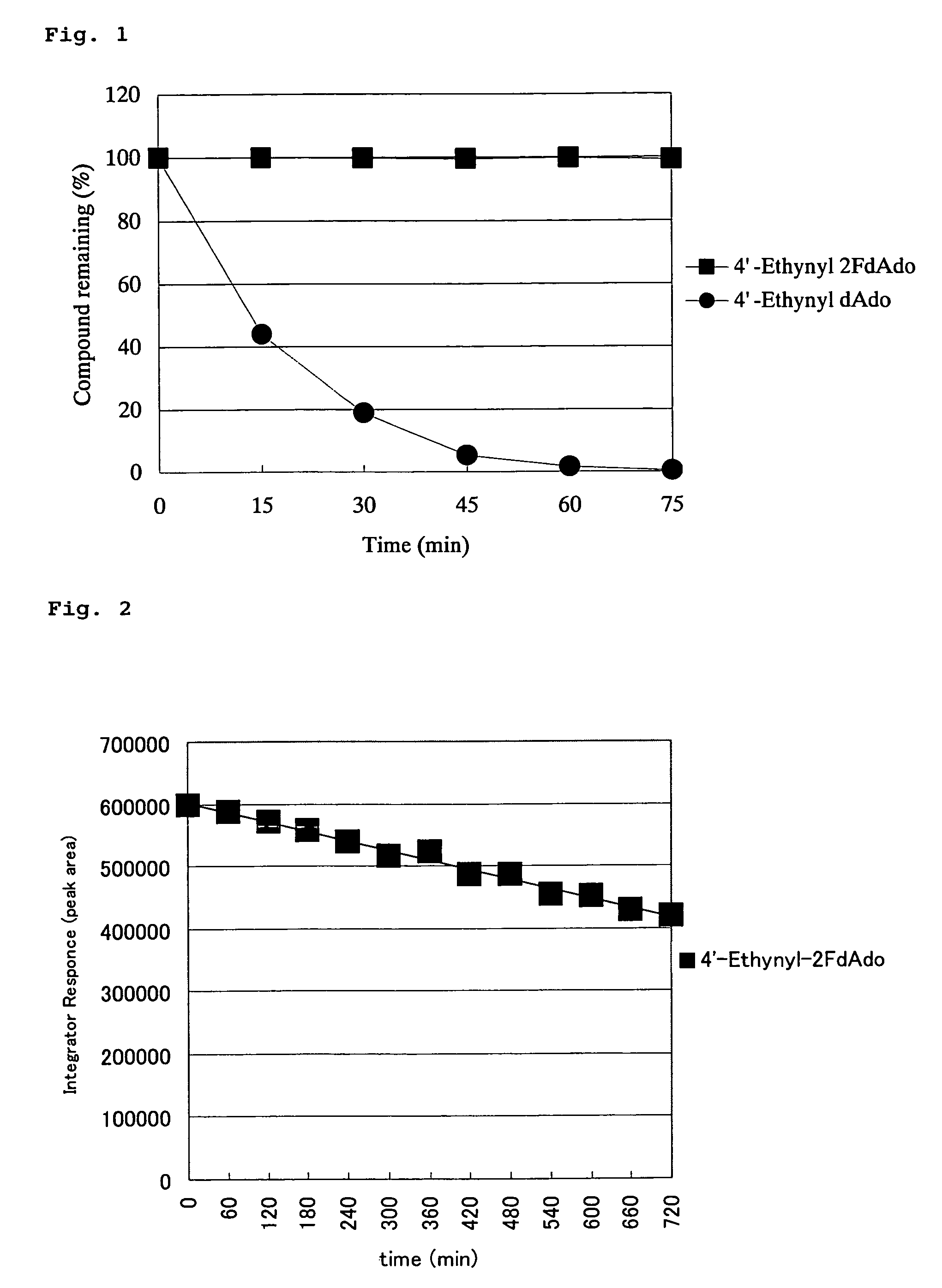
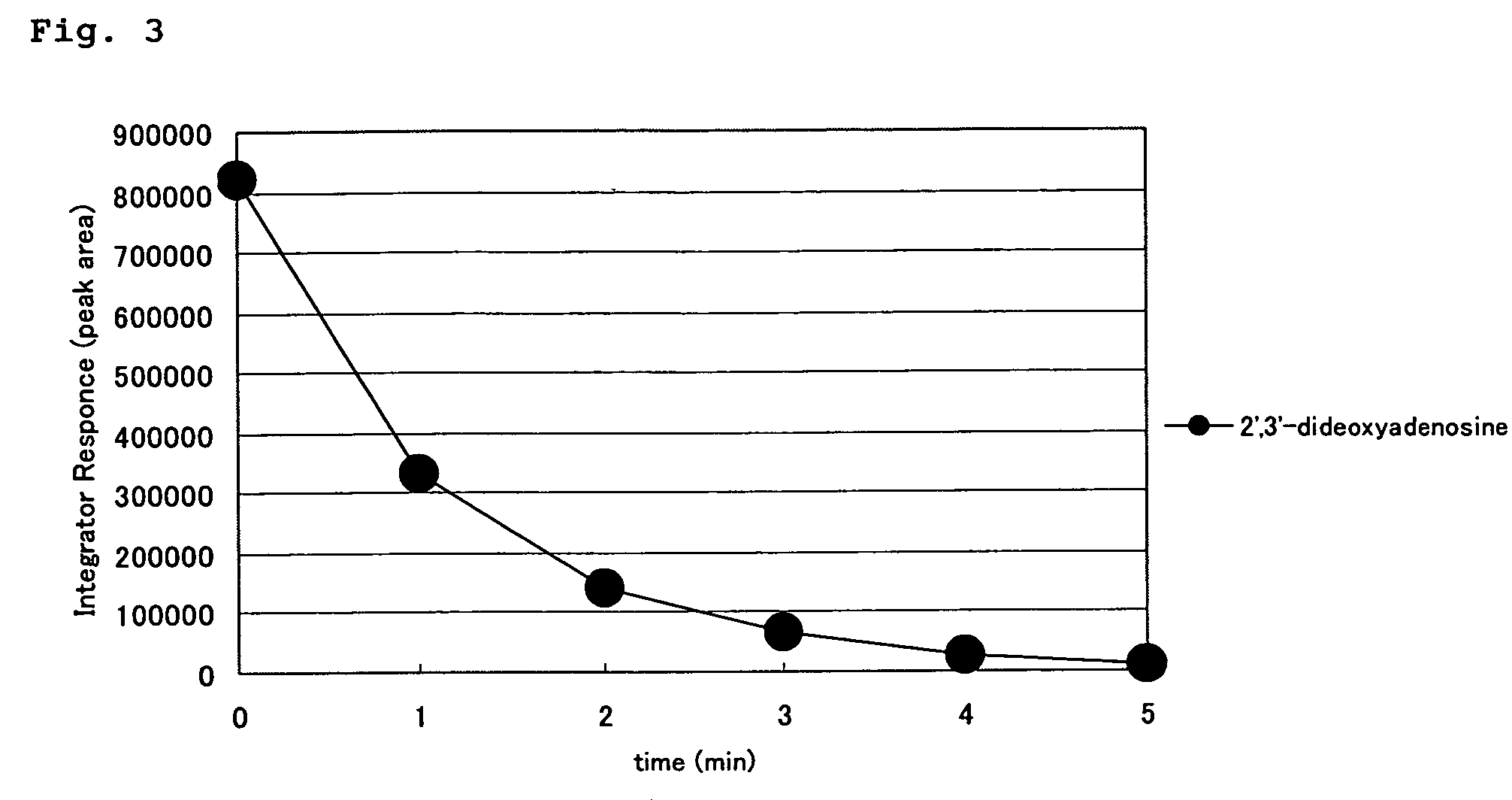
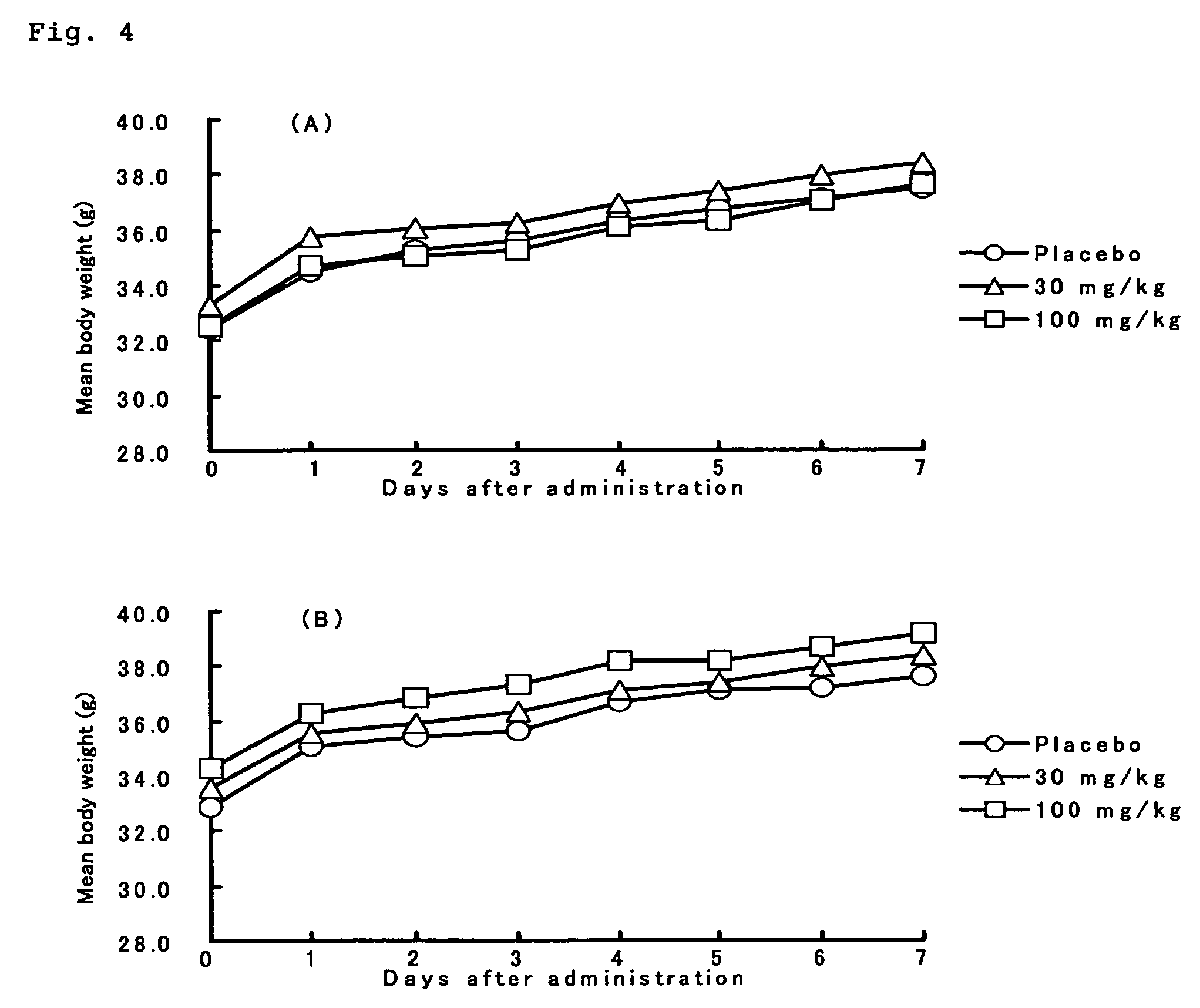
4′-C-substituted-2-haloadenosine derivative
a derivative and c-substitute technology, applied in the field of 4′csubstituted2haloadenosine derivatives, can solve the problems of not being able to achieve the effects of d4t, halogen atoms remain unknown, and the selectivity index cannot be improved, so as to achieve enhanced anti-hiv activity, anti-hiv activity is higher, and the effect of antiviral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
Synthesis of 2′-deoxy-4′-C-ethynyl-2-fluoroadenosine (Compound 4)
(1) Synthesis of 9-(3,5-di-O-acetyl-2-deoxy-4-C-ethynyl-β-D-ribo-pentofuranosyl)-2,6-diaminopurine (Compound 2)
[0126]
[0127]Compound 1 (0.33 g, 1.14 mmol) was suspended in acetonitrile (10.0 ml), and acetic anhydride (0.23 ml, 2.43 mmol), triethylamine (0.67 g, 4.81 mmol), and a small amount of 4-dimethylaminopyridine were added to the resultant suspension, followed by stirring at room temperature overnight.
[0128]The thus-precipitated crystals were filtered and dried, to thereby yield compound 2 (0.40 g, 1.07 mmol, 93.9%).
[0129]1H-NMR(DMSO-d6)δ7.94(1H, s, H-8), 6.76(2H, bs, NH2), 6.27(1H, t, H-1′, J=7.00), 5.84(2H, bs, NH2), 5.60(1H, dd, H-3′, J=4.00, 6.80), 4.46(1H, d, H-5′a, J=11.5), 4.21(1H, d, H-5′b, J=11.5), 3.74(1H, s, ethynyl) 3.12(1H, m, H-2′a), 2.52(1H, m, H-2′b), 2.12, 2.03(each 3H, s, acetyl)
(2) Synthesis of 3′, 5′-di-O-acetyl-2′-deoxy-4′-C-ethynyl-2-fluoroadenosine (Compound 3)
[0130]
[0131]Compound 2 (450 mg,...
synthesis example 2
Synthesis of 4′-C-cyano-2′-deoxy-2-fluoroadenosine (Compound 8)
(1) Synthesis of 9-(3,5-di-O-acetyl-4-C-cyano-2-deoxy-β-D-ribo-pentofuranosyl)-2,6-diaminopurine (Compound 6)
[0136]
[0137]Compound 5 (122 mg, 0.418 mmol) was suspended in acetonitrile (5.00 ml), and acetic anhydride (118 μl, 1.25 mmol), triethylamine (352 μl, 2.51 mmol), and a small amount of 4-dimethylaminopyridine were added to the resultant suspension, followed by stirring at room temperature overnight. The thus-precipitated crystals were filtered and dried, to thereby yield compound 6 (128 mg, 0.341 mmol, 81.6%).
[0138]1H-NMR(CDCl3) δ7.54(1H, s, H-8), 6.31(1H, t, H-1′, J=7.00), 6.06(1H, dd, H-3′, J=5.00, 6.50), 5.31(2H, bs, NH2), 4.95(1H, d, H-5′a, J=11.5), 4.80(2H, bs, NH2), 4.37(1H, d, H-5′b, J=12.0), 3.43(1H, m, H-2′a), 2.63(1H, m, H-2′b), 2.23, 2.12(each 3H, s, acetyl).
(2) Synthesis of 3′, 5′-di-O-acetyl-4′-C-cyano-2′-deoxy-2-fluoroadenosine (Compound 7)
[0139]
[0140]Compound 6 (118 mg, 0.314 mmol) was dissolved in 7...
synthesis example 3
Synthesis of 2-chloro-2′-deoxy-4′-C-ethynyladenosine (Compound 9)
[0145]
[0146]Benzyltriethylammonium nitrite (1.04 g, 4.36 mmol) was dissolved in dichloromethane (24.0 ml), and acetyl chloride (0.40 ml, 5.63 mmol) was added to the resultant solution, followed by stirring at 0° C. for 30 minutes. To the resultant solution, a solution of compound 2 (340 mg, 0.91 mmol) in dichloromethane (6.00 ml) was added, and the resultant mixture was stirred at 0° C. for three hours. The resultant reaction mixture was diluted with chloroform, and subsequently the resultant organic layer was washed with water, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. To the resultant residue, 28% aqueous ammonia (10.0 ml) and methanol (15.0 ml) were added, and the resultant mixture was stirred at room temperature overnight. Thereafter, the resultant reaction mixture was concentrated under reduced pressure, and the resultant residue was purified by means of silica gel column chr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com