Metal carbonate initiator and method for polymerizing isocyanates using the same
a technology of initiators and metal carbonates, which is applied in the direction of organic chemistry, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problems of difficult control of the molecular weight of a polymer and to obtain a monodispersed polymer, the method of controlling the polymerization of isocyanates is a very difficult problem, and the anionic polymerization of isocyanates requires extreme reaction conditions. to achiev
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
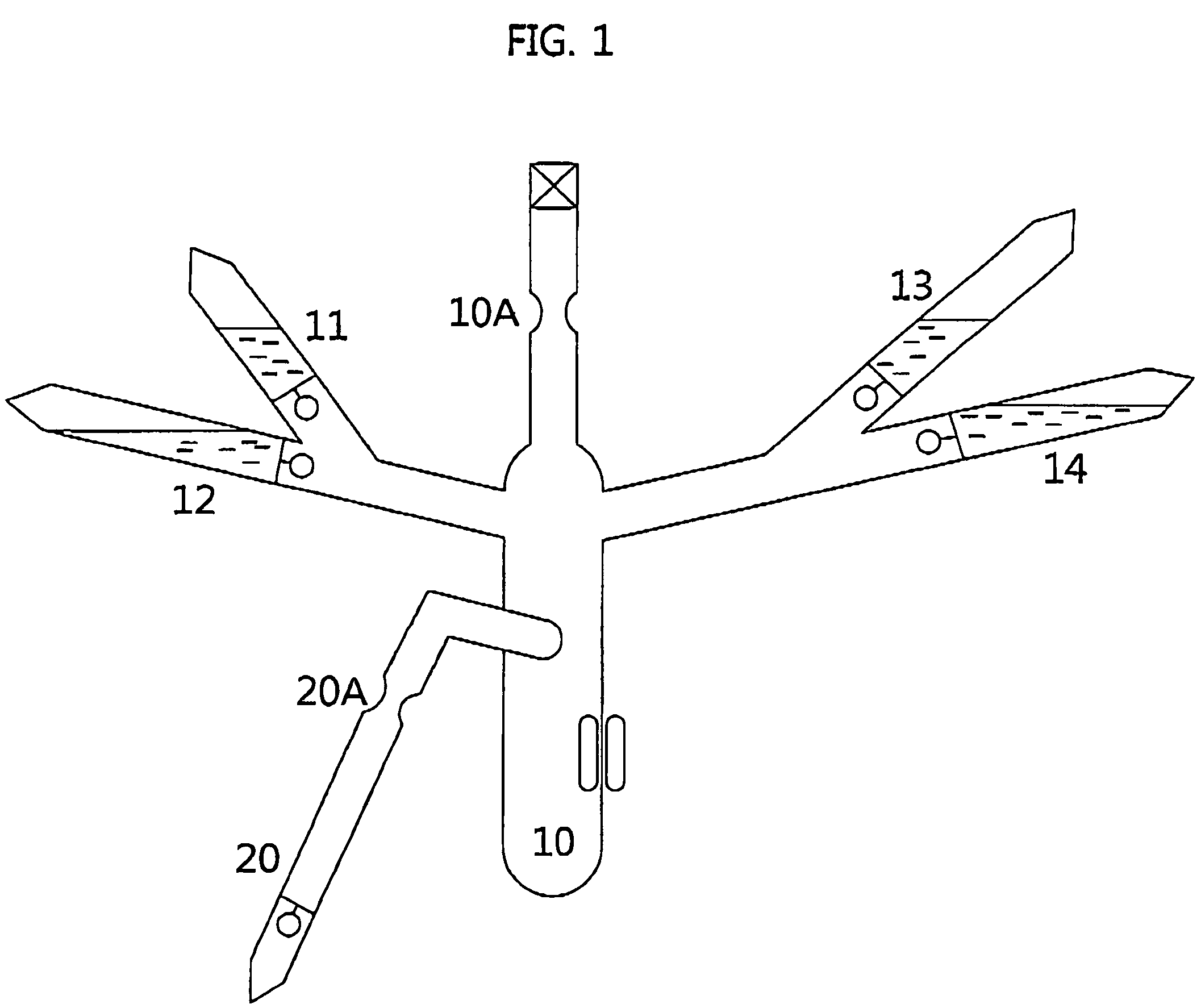
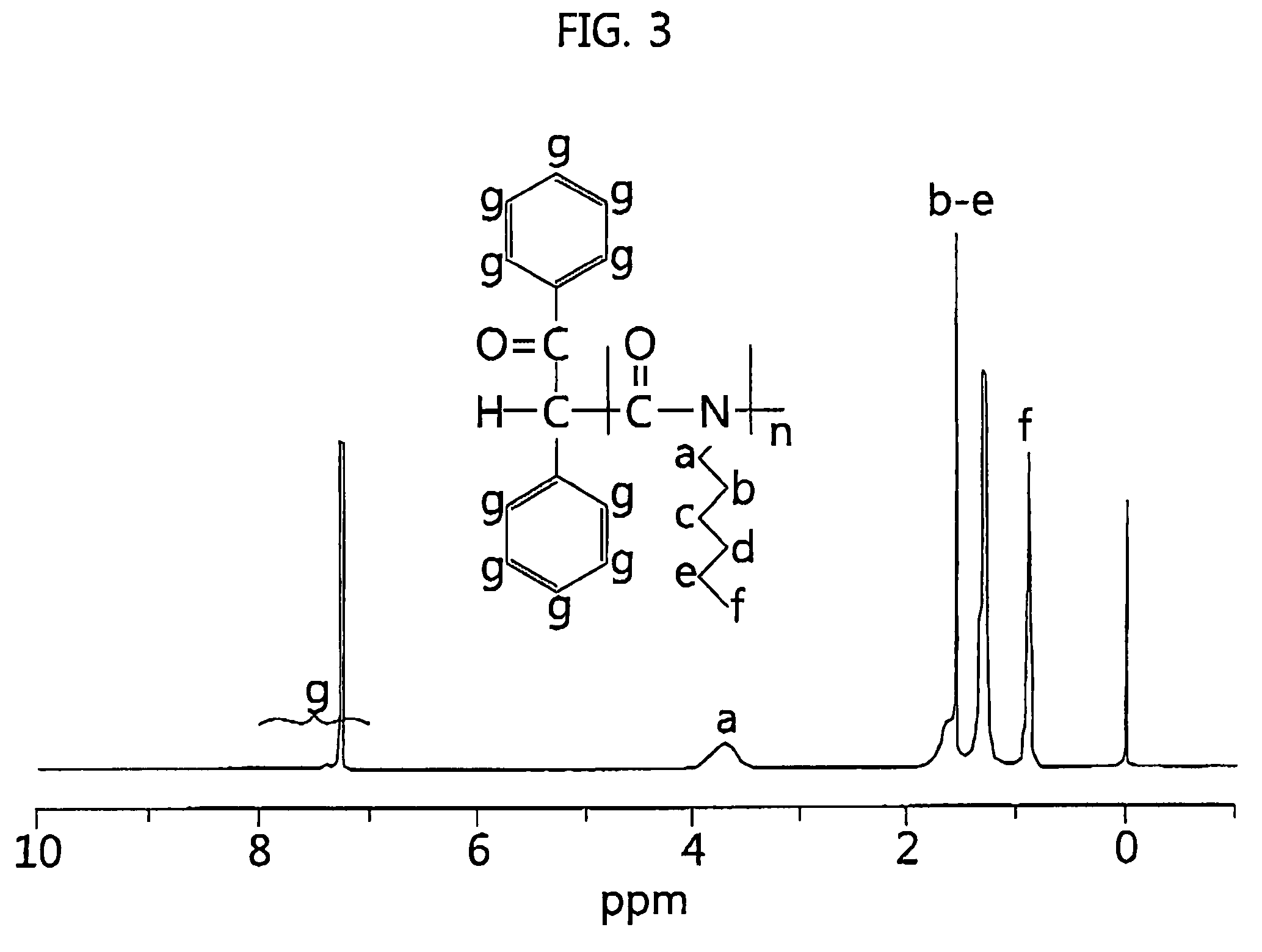
[0054]Poly(n-hexylisocyanate) was prepared using n-hexylisocyanate (HIC) shown in Table 1 in the following manner. The reaction conditions were −98° C. and 10−6 torr, and the reaction time was set to 5 to 15 minutes. To set the reaction temperature, liquid nitrogen was added to methanol contained in a constant temperature bath to freeze the methanol, and the temperature was measured using a low-temperature thermometer. Pale yellow sodium-deoxybenzoin (Na—DB) obtained by reacting sodium metal with an equivalent amount of deoxybenzoin in a tetrahydrofuran (THF) solvent was used as the initiator. The thus prepared initiator was immediately put into a glass ampoule under vacuum and then diluted to an appropriate concentration. A polymerization unit shown in FIG. 1, comprising the glass ampoule containing purified n-hexylisocyanate, i.e., an isocyanate monomer, the initiator prepared in the above-described manner, and a reaction terminator, was connected to a vacuum line to be in a high ...
example 2
[0057]Poly(n-hexylisocyanate) was prepared in the same manner as Example 1, except that the reaction molar ratio was differentiated as shown in the following Table 2 to examine the effects according to the change of the molecular weight during the polymerization. At this time, the reaction time was set to 10 minutes, which was the optimum reaction condition.
[0058]
TABLE 2PolymerizationNumber-average molecularPolydispersitycomponent (mmol)weight (Mn)indexYieldClassificationNa-DB a)HIC b)Calculated c)Measured d)(Mw / Mn)(%)Example 2-10.2728.644240115001.08100Example 2-20.25312.36330191001.0399Example 2-30.27017.88390245001.0598Example 2-40.23122.412300353001.0798a) Initiator: sodium-deoxybenzoinb) Isocyanate monomer: n-hexylisocyanatec) Calculated value: {(monomer content / initiator content) × molecular weight of hexylisocyanate (127.19) + molecular weight of deoxybenzoin (196.25)} × polymer yield / 100d) Measured value: measured at 40° C. using gel permeation chromatography (GPC) and light...
example 3
[0059]Poly(3-(triethoxysilyl)propyl isocyanate) was prepared in the same manner as Example 1, except that 3-(triethoxysilyl)propyl isocyanate (TESPI) was used. At this time, the reaction time was set to 5 to 12 minutes, and the reaction was terminated by methanol. The thus prepared polymer was precipitated in excess methanol and then vacuum-dried.
[0060]
TABLE 3PolymerizationReactionNumber-average molecularPolydispersitycomponent (mmol)timeweight (Mn)indexYieldClassificationNa-DB a)TESPI b)(min)Calculated c)Measured d)(Mw / Mn)(%)Example 3-10.2423.8352840104001.1369 (31) e)Example 3-20.2423.8383990107001.1197Example 3-30.2443.97103800113001.1190 (10) f)Example 3-40.2453.69123140107001.1280 (20) f)a) Initiator: sodium-deoxybenzoinb) Isocyanate monomer: 3-(triethoxysilyl)propyl isocyanatec) Calculated value: {(monomer content / initiator content) × molecular weight of 3-(triethoxysilyl)propyl isocyanate (247.37) + molecular weight of deoxybenzoin (196.25)} × polymer yield / 100d) Measured val...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com