Method and apparatus for transdermal or transmucosal application of testosterone
a technology of testosterone and transdermal or transmucosal injection, which is applied in the direction of biocide, plant growth regulator, pharmaceutical non-active ingredients, etc., can solve the problems of lack of sex drive, clinical symptoms, and undesirable clinical symptoms, and achieve effective treatment, reduce or alleviate clinical symptoms, and pain-free and pain-free effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
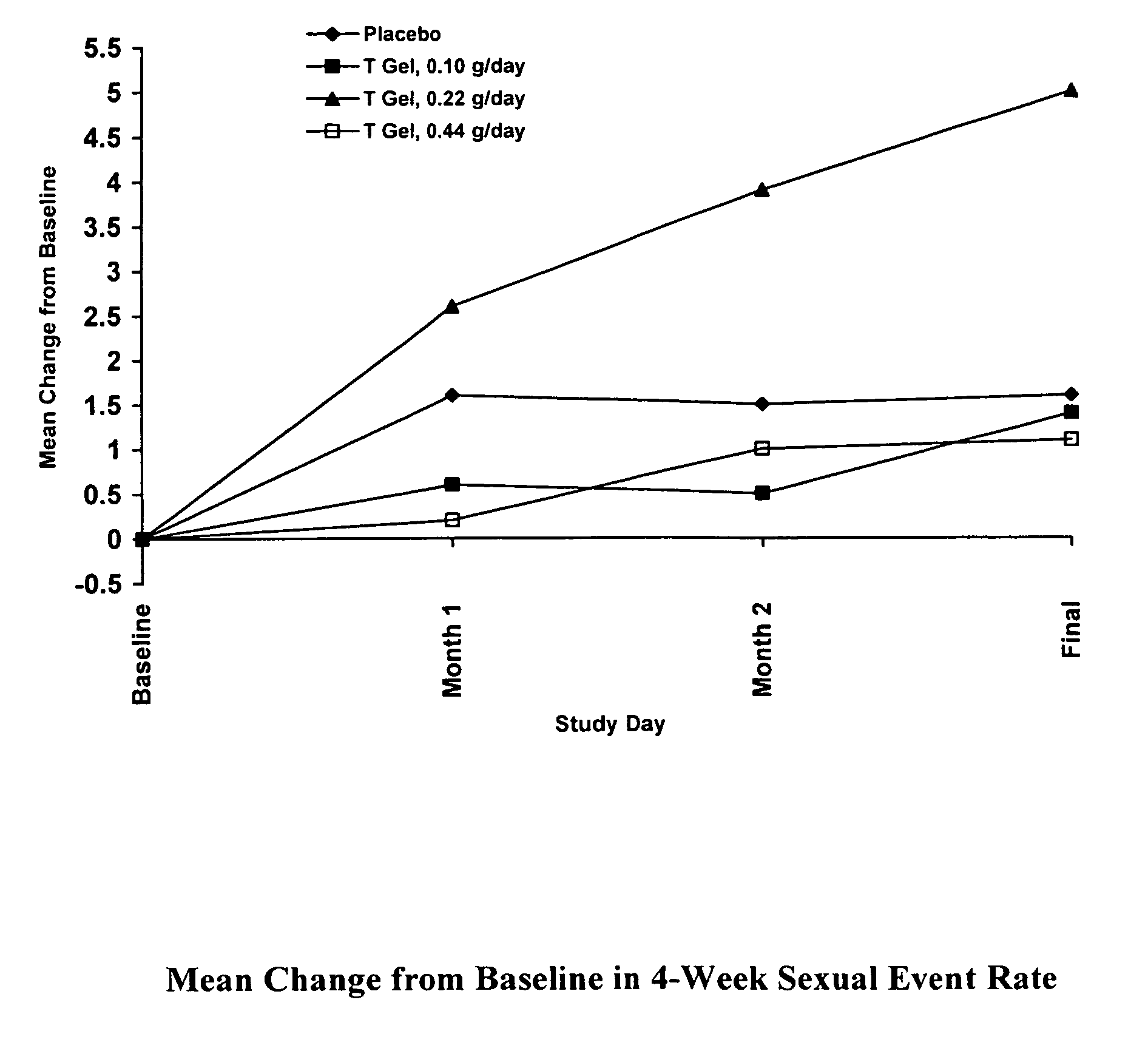
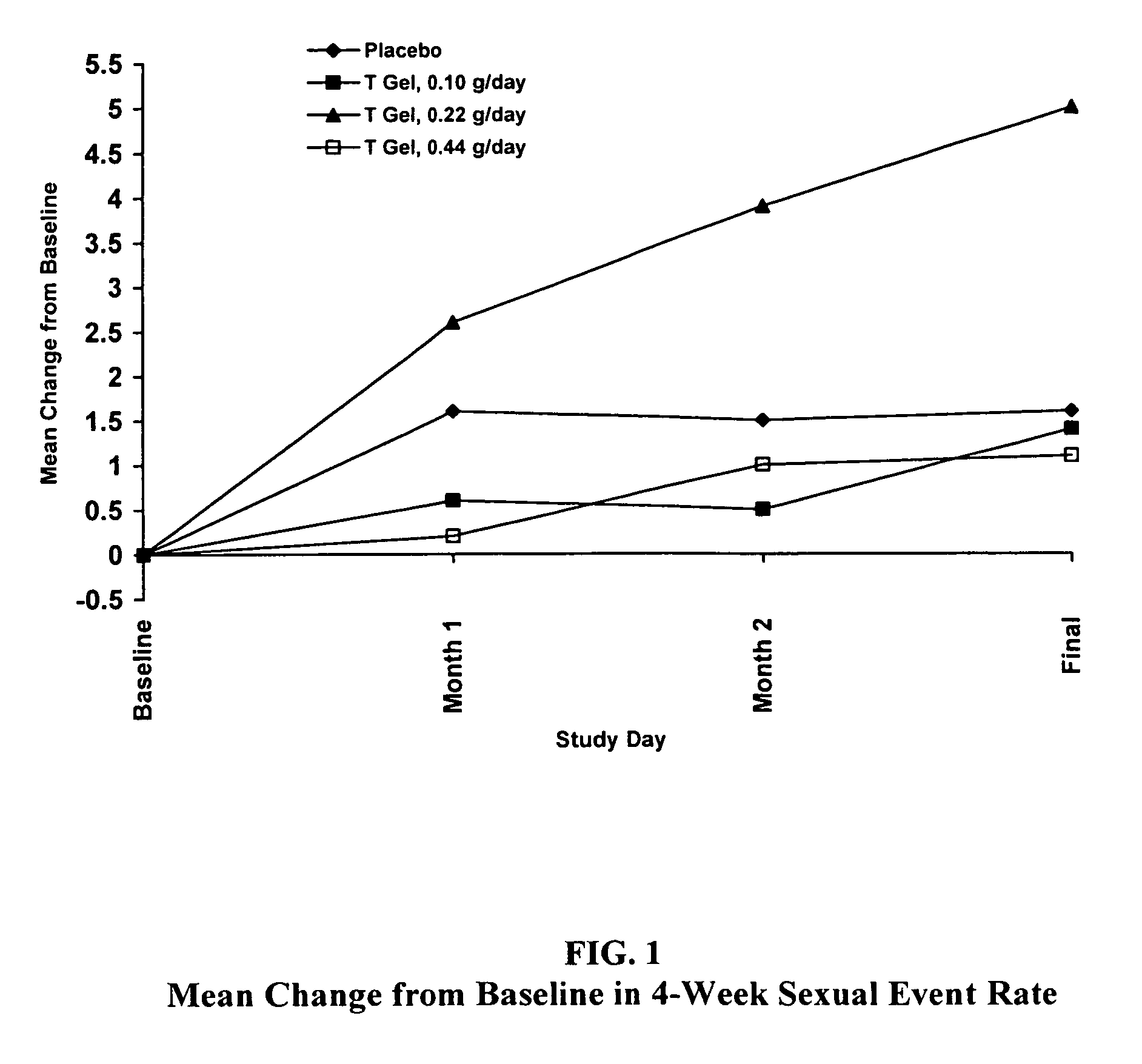
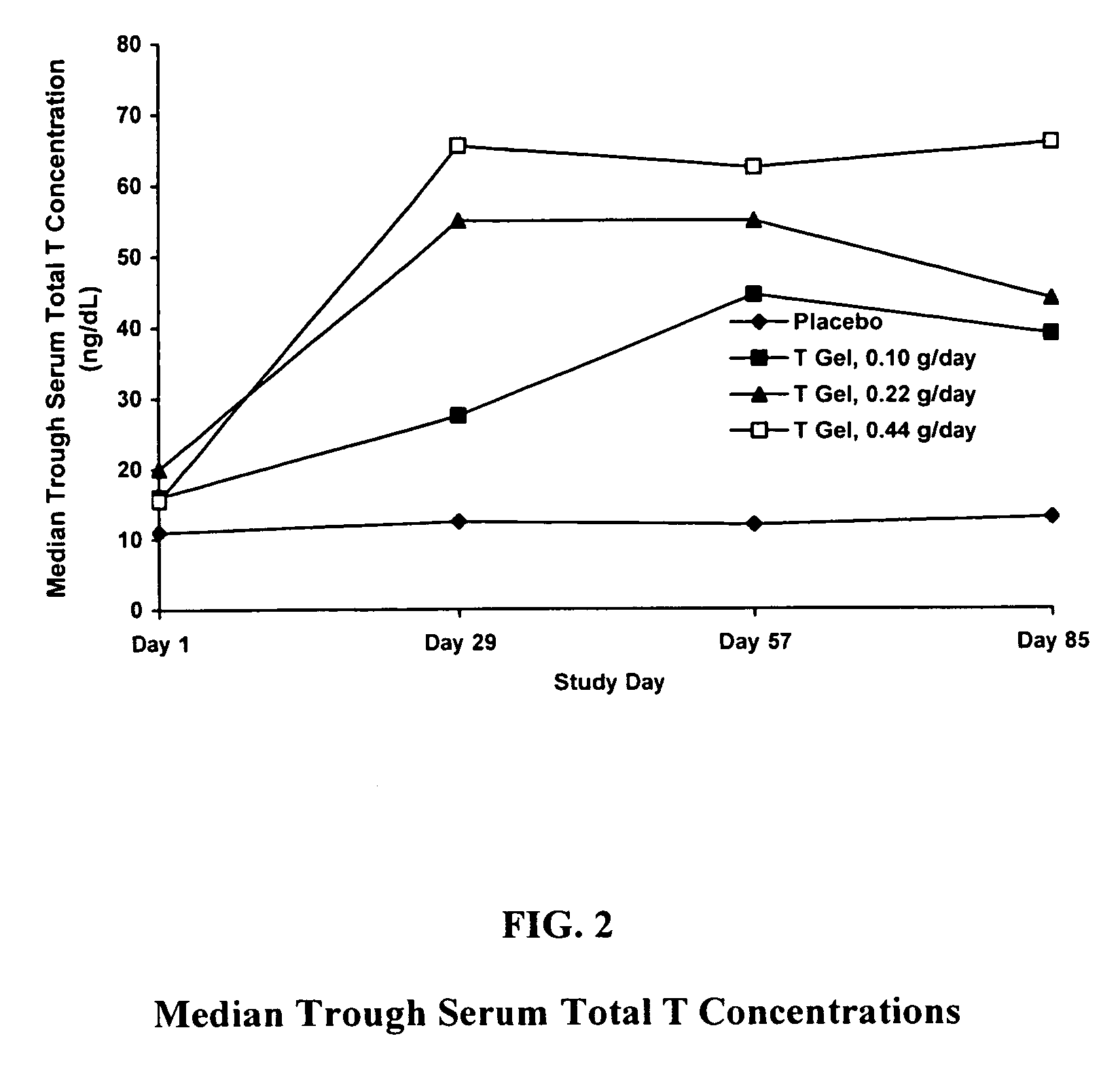
Comparative Study of T Gel Formulation Doses
[0072]To test the efficacy of the present gel formulation delivery, a comparative study was designed to detect a clinically significant difference in serum T concentration and 4-week satisfying sexual event rate. The study was conducted with 46 surgically menopausal women with a serum free T≦1.5 pg / mL, who were on a stable dose of conjugated estrogen of at least 0.625 mg / day or an equivalent oral estrogen for at least two months, and consisted of an 8-week pretreatment period and a 12-week double-blind treatment period (Days 1 to 85). At Day 1, eligible subjects were equally randomized to one of four treatment arms: 0.10 g / day (1.0 mg T / day), 0.22 g / day (2.2 mg T / day), or 0.44 g / day (4.4 mg T / day) of the present gel formulation with 1% testosterone (hereafter denoted as “T Gel”), or a matching placebo gel. Subjects returned for safety and efficacy evaluations every 4 weeks, and serum trough hormone samples were drawn at each visit. The fol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com