Intranasal delivery of therapeutic enzymes to the central nervous system for the treatment of lysosomal storage diseases
a technology of lysosomal storage and therapeutic enzymes, which is applied in the direction of peptide/protein ingredients, drug compositions, enzymology, etc., can solve the problems of relatively large inability to facilitate or achieve delivery, and achieve the effect of maximizing the amount of enzymes reaching the brain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
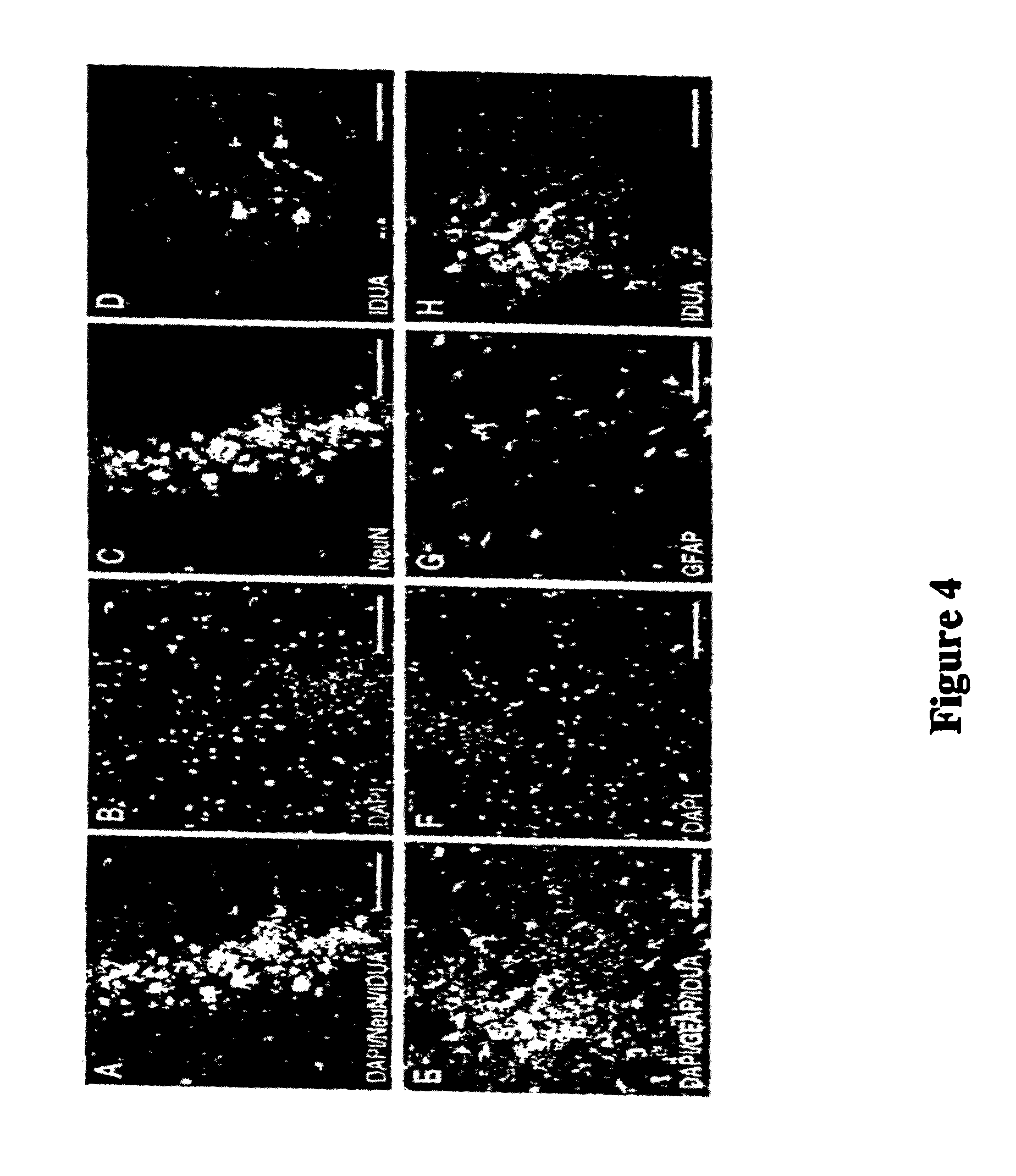
Intranasal Administration of IDUA Leads to Widespread Distribution in the Brains of Mice
[0076]IDUA− / − mice were anesthetized and treated with either 24 μL of PBS vehicle or laronidase (Aldurazyme) that had been concentrated by centrifugation in an Amicon Centriplus YM-10 column to a final concentration of about 1.5 mg / mL. Prior to concentration, the laronidase was diluted with two parts Elliots B solution (which is comparable in pH, electrolyte composition, glucose content, and osmolarity to cerebrospinal fluid, and includes sodium chloride, sodium bicarbonate, dextrose, magnesium sulfate, potassium chloride, calcium chloride, and sodium phosphate, resulting in sodium 149 mEq / liter, potassium 4.0 mEq / liter, calcium 2.7 mEq / liter, magnesium 2.4 mEq / liter, bicarbonate 22.6 mEq / liter, chloride 132 mEq / liter, sulfate 2.4 mEq / liter, and phosphate 1.5 mEq / liter). The treatments were administered by applying a series of eight 3 μL drops to the nasal cavity of each mouse at one-minute inter...
example ii
Testing the Effectiveness of Enzyme Delivery to the CNS
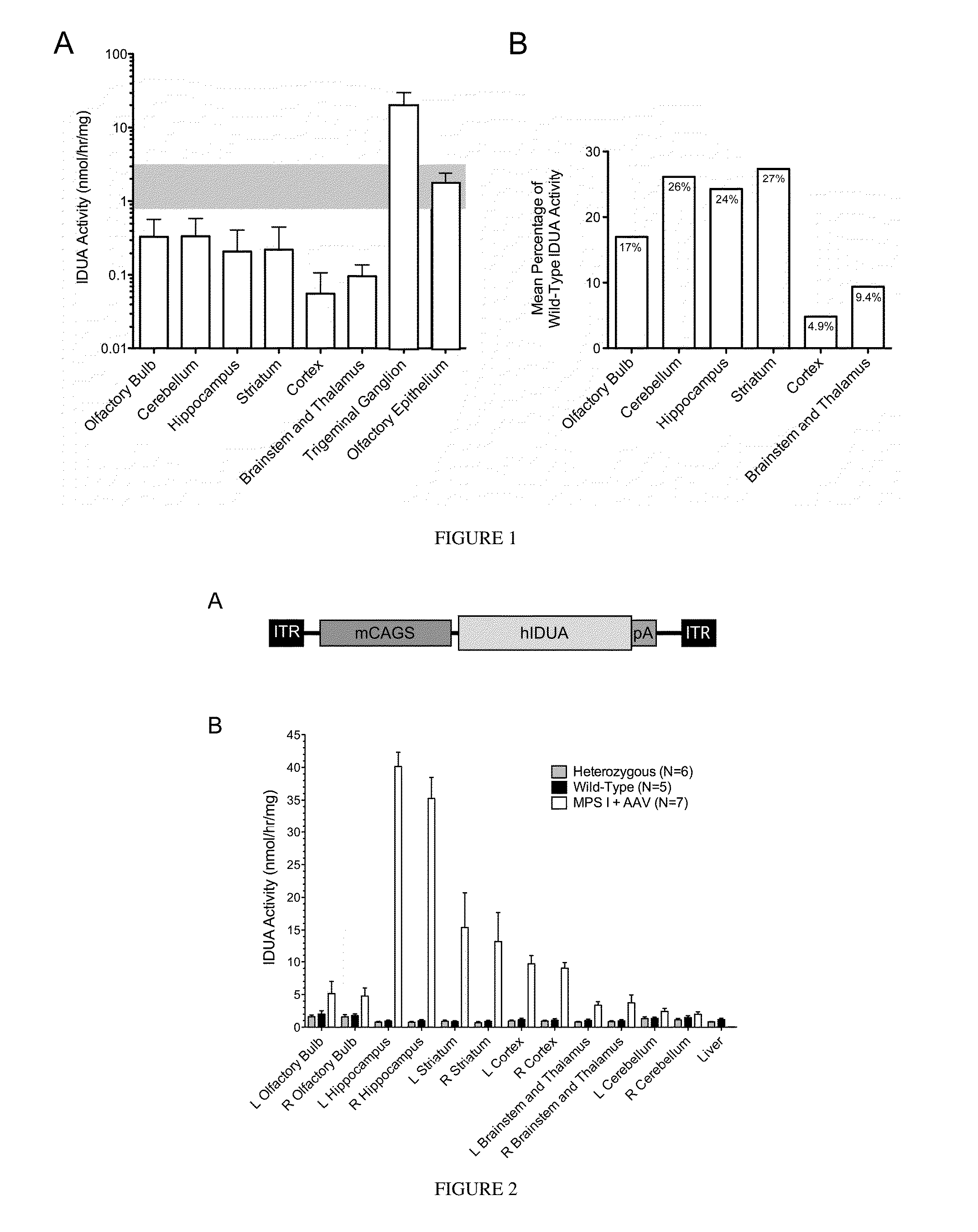
[0078]The effectiveness of enzyme delivery to the CNS is described in the following examples, in which iduronidase enzyme was expressed in all sections of the brain after delivery of adeno-associated virus vector encoding human IDUA.
[0079]An AAV 2 based vector (AAV2-MCI) was constructed to contain the human IDUA (hIDUA) coding sequence under regulation of a strong mini-CAGS promoter (Ohlfest et al., 2005) (FIG. 2A). This vector was packaged at the University of Florida Vector Core using AAV 8 capsid protein, and 2×1010 vector genomes was stereotactically infused into the right lateral ventricle of neonatal (4-6 day old) IDUA-deficient mice, anticipating that this route of administration would provide widespread distribution and vector-mediated hIDUA expression throughout the brain.
[0080]The animals were sacrificed at 10 months of age, and brain tissue was harvested by microdissection and homogenized. IDUA was undetectable in bra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com