Method of using prourokinase in facilitated percutaneous coronary intervention in patients with acute myocardial infarction
a percutaneous coronary intervention and prourokinase technology, applied in the field of biological medicines, can solve the problems of high poor efficacy and safety of facilitated pci by using presently approved thrombolytic drugs, and patients cannot receive direct pci therapy, so as to prevent hemorrhagic complications, reduce the incidence rate of reinfarction after thrombolysis, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 3
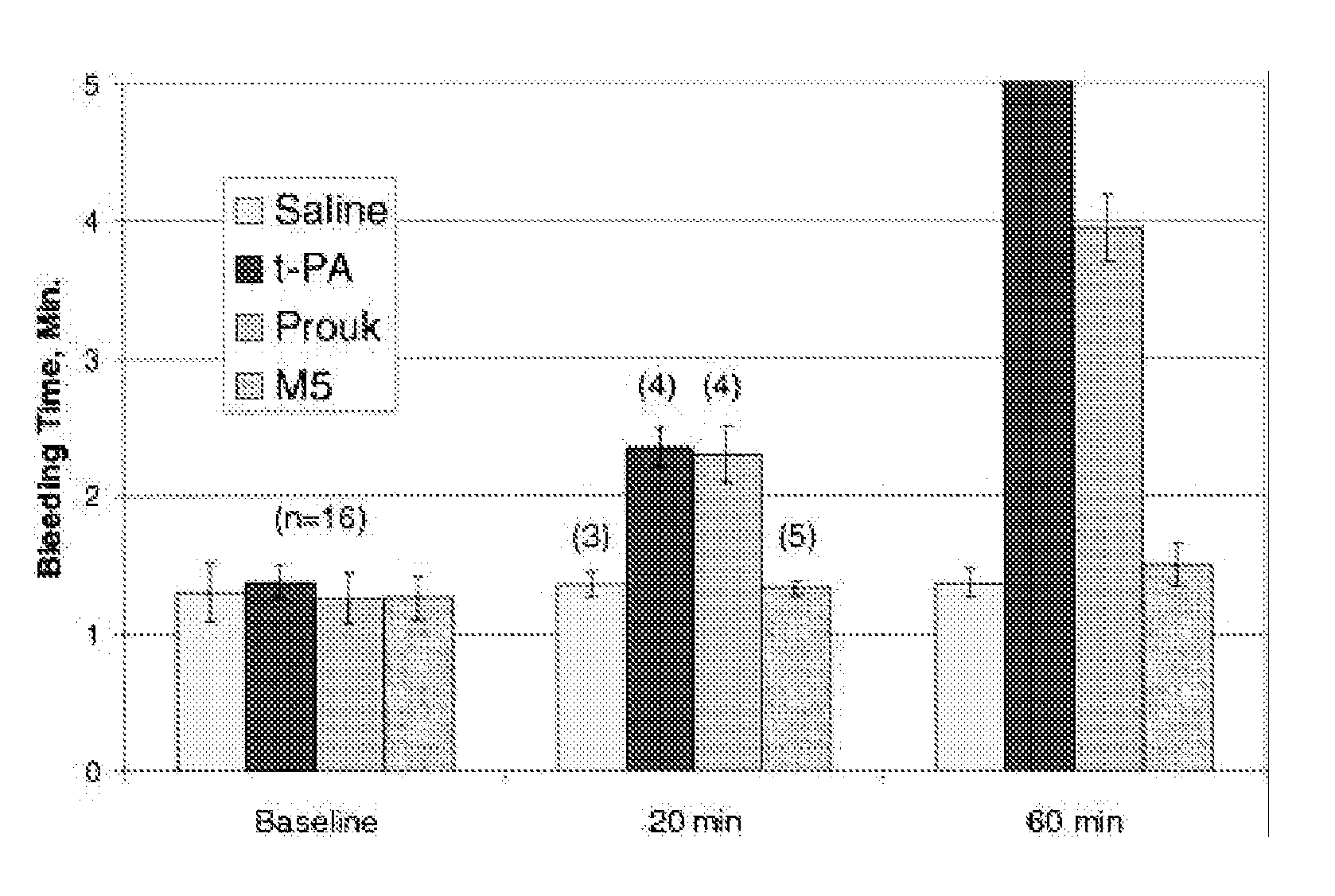
Application of ProUK in Facilitated PCI
[0058](1) Selection Criteria
[0059]1.1 Ischemic chest pain lasting for 30 min or more, and sublingual administration of nitroglycerin being ineffective;
[0060]1.2 Sustained ischemic chest pain lasting for 12 hrs or less;
[0061]1.3 Electrocardiogram (ECG) having at least two or more ST segment elevations of 0.1 mV or more in limb lead, or two or more adjacent ST segment elevations of 0.2 mV or more in chest lead; and
[0062]1.4 Age of 85 years old or less, male or female.
[0063](2) Exclusion Criterion
[0064]2.1 Non-ST segment-elevation AMI or unstable angina pectoris;
[0065]2.2 Women during pregnant stage, breast-feed stage, and menstrual period;
[0066]2.3 Hemorrhagic stroke occurred at anytime in the past, and ischemic stroke or cerebrovascular event occurred in 1 year;
[0067]2.4 Severe progressive diseases (such as malignant tumor) or diseases with poor prognosis and making the patient to be extremely exhausted;
[0068]2.5 Active visceral hemorrhage (such...
PUM
| Property | Measurement | Unit |
|---|---|---|
| delay time | aaaaa | aaaaa |
| delay time | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com