Method of hemodilution facilitated by monitoring oxygenation status
a technology of oxygenation status and hemoglobin level, which is applied in the direction of drug compositions, peptide/protein ingredients, extracellular fluid disorder, etc., can solve the problems of large volume of blood substitutes used in transfusions, insufficient use of autologous predonation, and ineffective use of fluosol in sever anemia, etc., to reduce the patient's blood hemoglobin level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Enhancement of O.sub.2 Delivery by Perfluorocarbon Emulsion
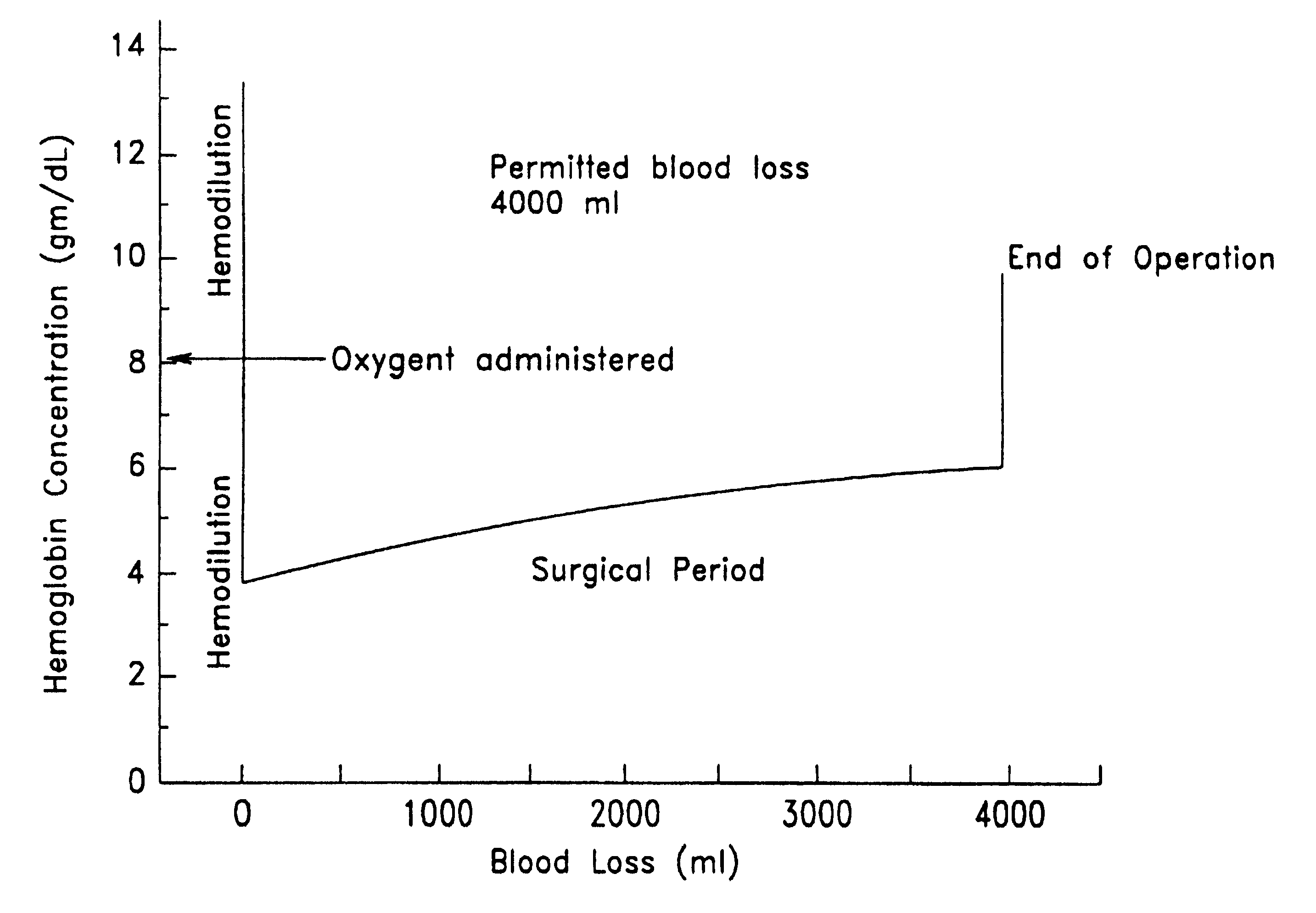
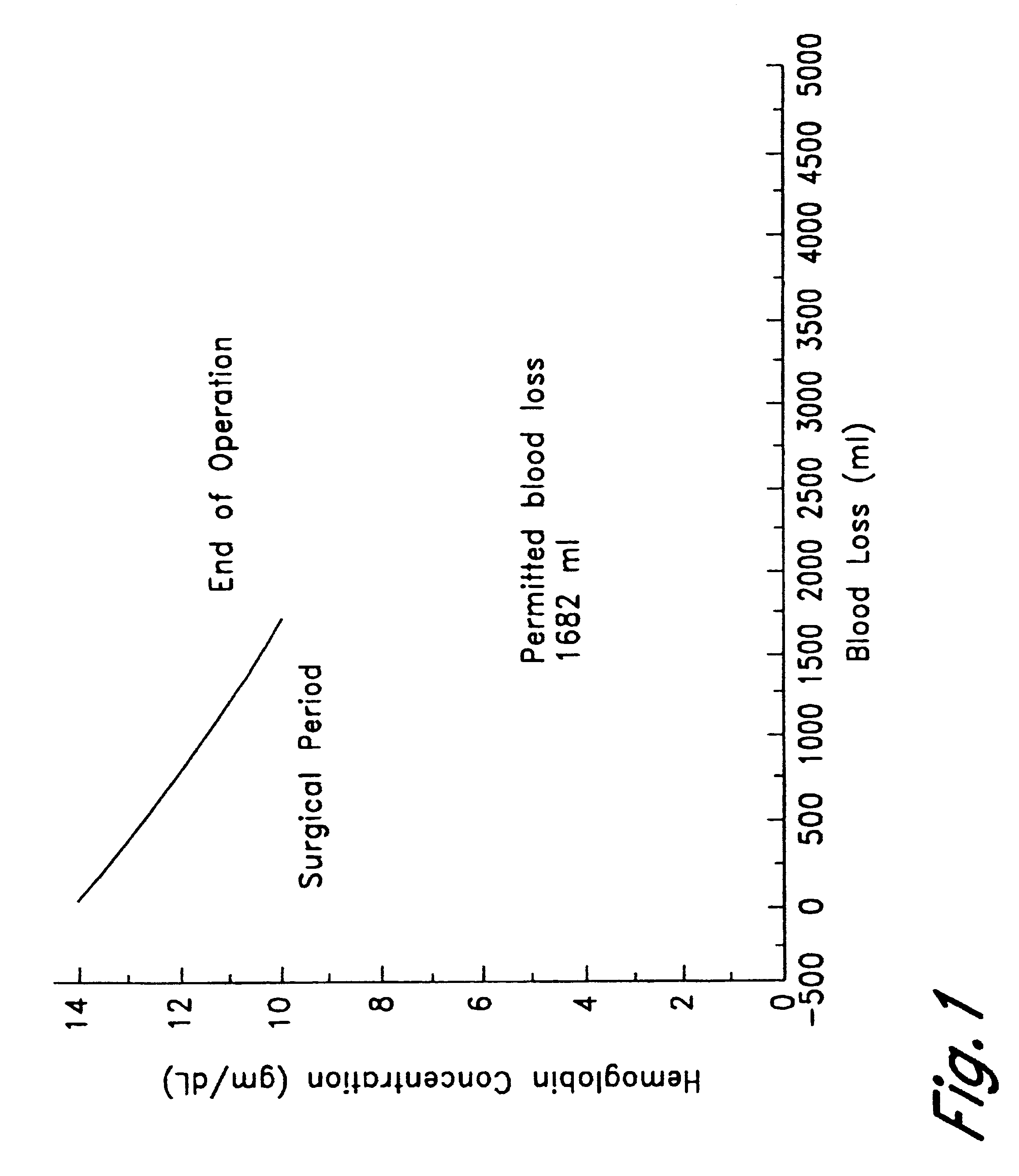
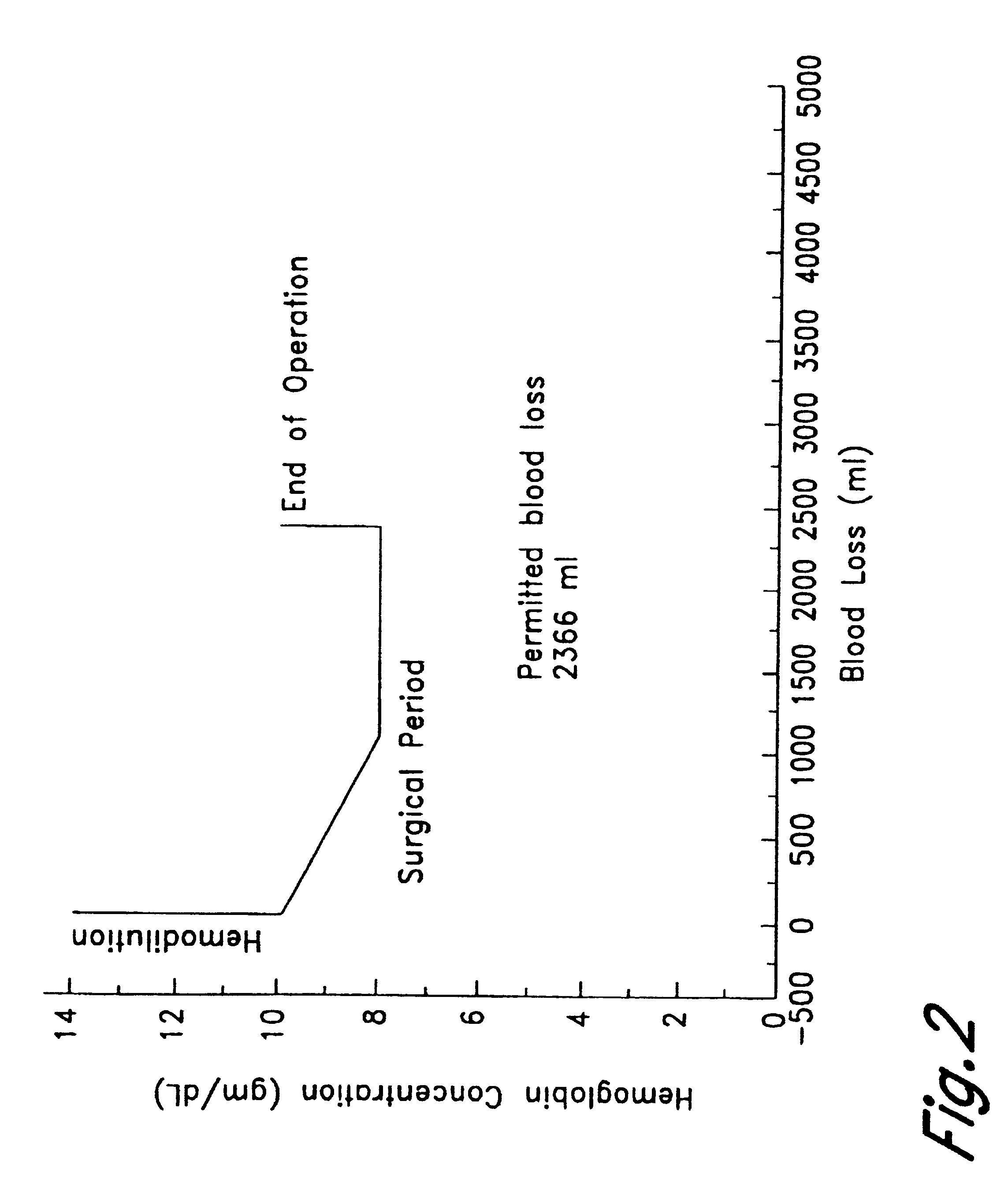
Immediately prior to undergoing surgery, a patient is subjected to perioperative isovolemic hemodilution. The removed blood is stored for later use. Blood is removed with the concomitant intravenous replacement by a crystalloid solution. During this time, the patient's fractional inspired oxygen concentration (FiO.sub.2) is increased to 1. The patient is hemodiluted until the hemoglobin concentration reaches 8 gm / dL, with each aliquot of the removed blood being replaced by 3 volumes of Ringer-lactate. A 90% w / v perflubron emulsion having the composition of Formula I is administered intravenously to a total dose of 1.8 gm / kg body weight, while the patient's PvO.sub.2 is monitored using a Swan-Ganz catheter. Hemodilution and administration of perflubron emulsion is continued until the PvO.sub.2 reaches 40 mm Hg (hemoglobin level is 2 gm / dL). Surgery is then initiated, with an attendant blood loss of up to 3 liters. Autologous ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com