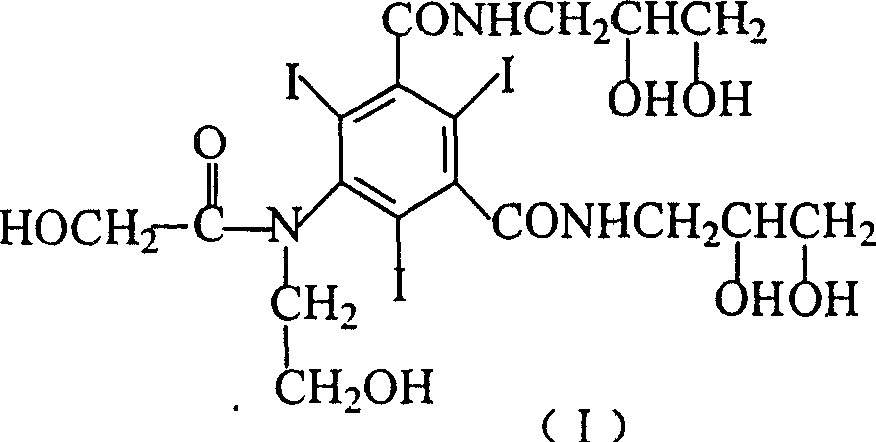
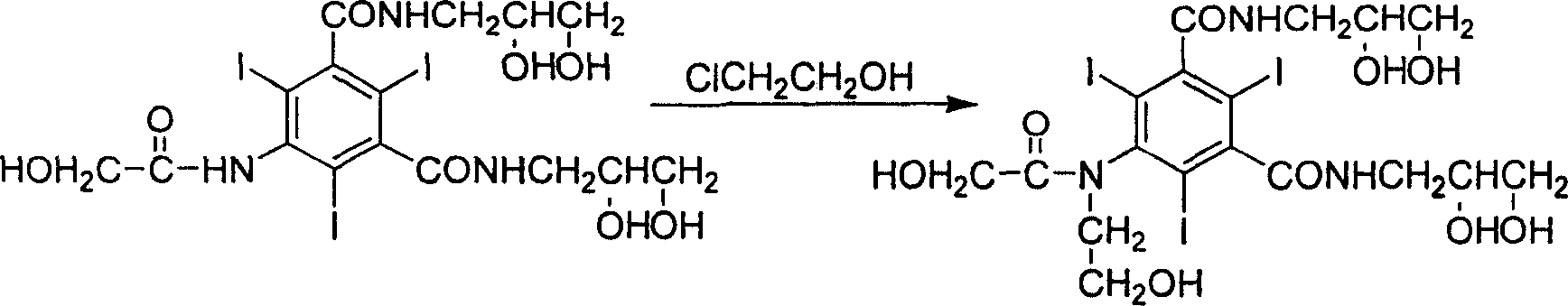
Improved process for synthesizing ioversol
A synthesis method and technology of ioversol are applied in the improved synthesis field of ioversol, can solve the problems such as inability to meet the needs of industrial production, difficulty in recovering solvent, etc., and achieve the effects of low cost, easy recovery of solvent and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The synthesis of embodiment 1 ioversol:
[0020] In a three-necked flask equipped with a stirrer and a reflux condenser, add 152.6 g (0.2 mol) of 5-hydroxyacetamido-N, N'-bis(2,3-dihydroxypropyl)-2,4 , 6-triiodo-1,3-benzenedicarboxamide, 24g (0.6moL) sodium hydroxide and 600mL water, after stirring and dissolving, add 200mL acetonitrile, stir for 5 minutes, then add 96.6g (1.2moL) 2 -chloroethanol, then warming up to 50°C, stirring for 6 hours to stop the reaction. Neutralize with dilute hydrochloric acid, then distill off the solvent under reduced pressure. The residue was dissolved in 300mL of methanol, filtered, the filtrate was evaporated to remove methanol, and the residue was dissolved in 500mL of water. The solution was treated with 732 cation exchange resin and 717 anion exchange resin to remove cations and anions in the solution. The solvent was evaporated under reduced pressure to obtain a residue, which was dried in vacuo to obtain 155 g of a white solid (...
Embodiment 2
[0022] The synthesis of embodiment 2 ioversol:
[0023] In a three-necked flask equipped with a stirrer and a reflux condenser, add 152.6 g (0.2 mol) of 5-hydroxyacetamido-N, N'-bis(2,3-dihydroxypropyl)-2,4 , 6-triiodo-1,3-benzenedicarboxamide, 16g (0.4moL) sodium hydroxide and 600mL water, after stirring and dissolving, add 200mL acetonitrile, stir for 5 minutes, then add 64.4g (0.8moL) 2 -chloroethanol, then warming up to 30°C, stirring for 4 hours to stop the reaction. Neutralize with dilute hydrochloric acid, then distill off the solvent under reduced pressure. The residue was dissolved in 300mL of methanol, filtered, the filtrate was evaporated to remove methanol, and the residue was dissolved in 500mL of water. The solution was treated with 732 cation exchange resin and 717 anion exchange resin to remove cations and anions in the solution. The solvent was evaporated under reduced pressure to obtain a residue, which was dried in vacuo to obtain 145 g of a white solid (...
Embodiment 3
[0025] The synthesis of embodiment 3 ioversol:
[0026] In a three-necked flask equipped with a stirrer and a reflux condenser, add 152.6 g (0.2 mol) of 5-hydroxyacetamido-N, N'-bis(2,3-dihydroxypropyl)-2,4 , 6-triiodo-1,3-benzenedicarboxamide, 32g (0.8moL) sodium hydroxide and 600mL water, after stirring and dissolving, add 200mL acetonitrile, stir for 5 minutes, then add 128.8g (1.6moL) 2 -chloroethanol, then warming up to 70°C, stirring for 8 hours to stop the reaction. Neutralize with dilute hydrochloric acid, then distill off the solvent under reduced pressure. The residue was dissolved in 300mL of methanol, filtered, the filtrate was evaporated to remove methanol, and the residue was dissolved in 500mL of water. The solution was treated with 732 cation exchange resin and 717 anion exchange resin to remove cations and anions in the solution. The solvent was distilled off under reduced pressure to obtain a residue, which was dried in vacuo to obtain 148 g of a white sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com