Method for separating L-amino-acid oxidase from venin
A technology of amino acid and oxidase, which is applied in the field of separation of L-amino acid oxidase, can solve problems such as the influence of L-amino acid oxidase enzyme activity, and achieve the effects of easy promotion, simple method, and avoiding the influence of enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
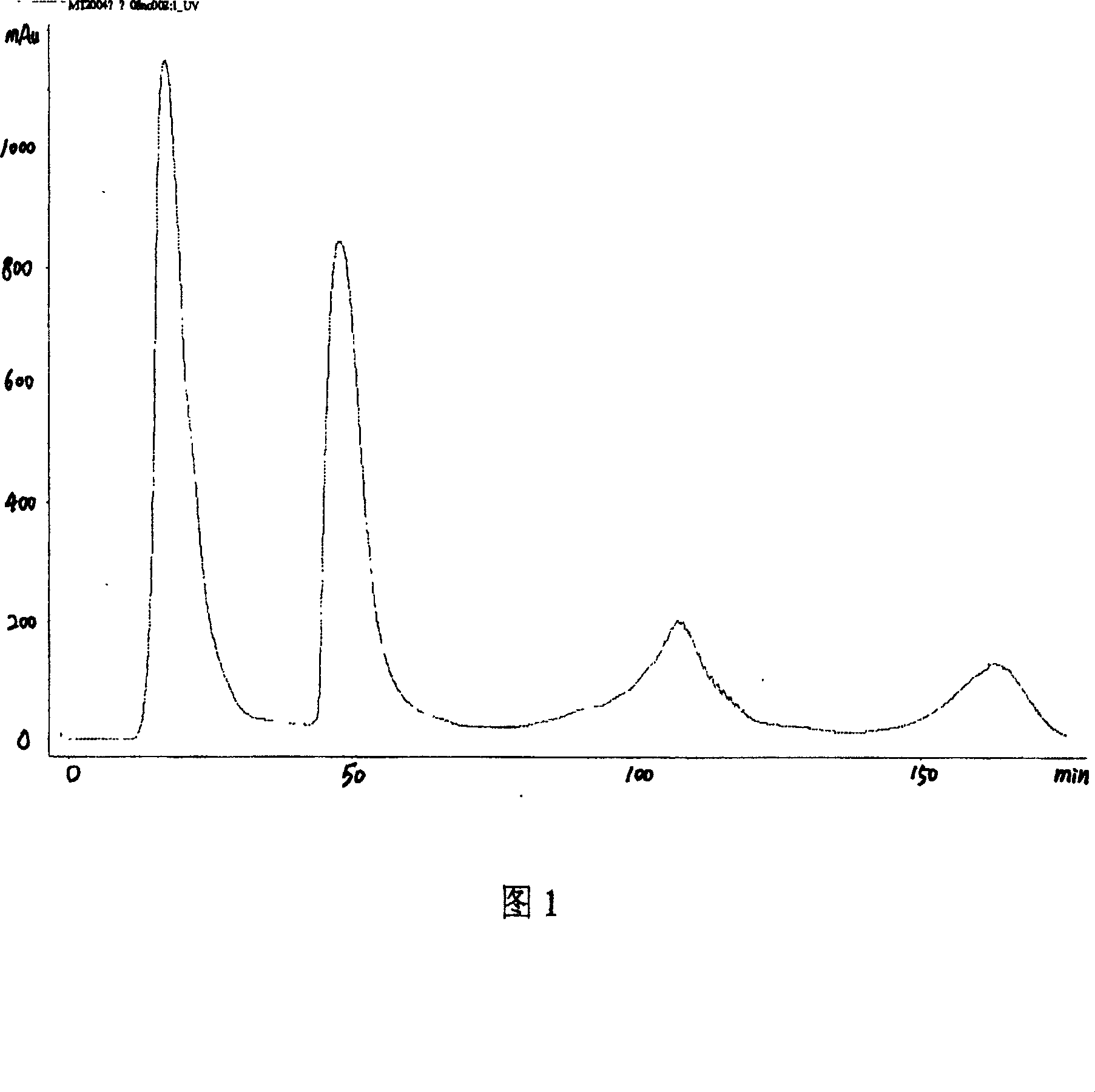
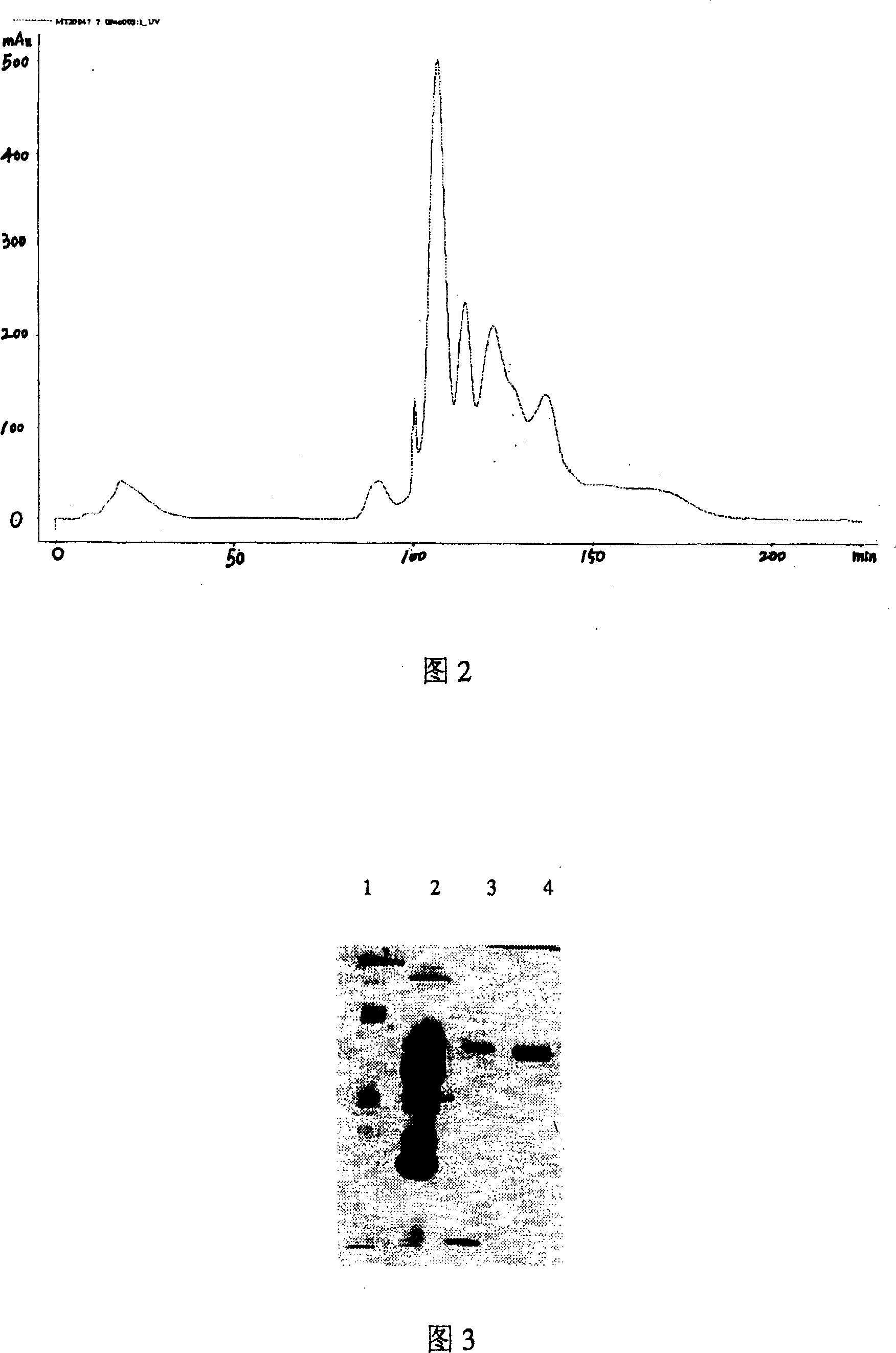
[0031] 1. Heparin affinity chromatography
[0032] (1) Sample processing: Weigh 200 mg of Agkistrodon halys venom, dissolve it in 5 mL of 25 mmol / LTris-HCl buffer solution (pH 8.5), and centrifuge at 10,000 r / min for 10 min at 4°C. Take the supernatant.
[0033] (2) Column packing: the volume of the chromatographic column is 25ml, and the filler is Heparin-Sepharose Fast Flow. Mobile phase A liquid is 25mmol / L Tris-HCl buffer solution (pH8.5), and B liquid is A liquid containing 1mol / L NaCl. When the liquid A is fully balanced until the conductivity value does not change, the snake venom sample is applied.
[0034] (3) Elution: After 50 mL of solution A, 250 mL was eluted with a linear gradient from 0% B to 100% B, and then 50 mL of column was washed with 100% B; the flow rate was 2 mL / min.
[0035] (4) Collection and detection: collect the eluate with a step-by-step collector, collect 3mL in each tube, and detect the protein content under the light absorption of 280nm. Th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com